Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Drug retention and response rates of TNFi treatment in 13,170 patients with psoriatic arthritis treated in routine care – pooled data from the EuroSpA Research Network Collaboration
Background/Purpose:
A research network collaboration of 15 European registries sharing data on patients with spondyloarthritis (SpA), “EuroSpA”, has recently been created to strengthen research capabilities in the real world setting1.
We aimed to investigate Tumour Necrosis Factor inhibitor (TNFi) retention and response rates at 6, 12 and 24 months in patients with psoriatic arthritis (PsA) treated with their 1st, 2nd or 3rd TNFi in clinical practice across Europe.
Methods:
A common data model was agreed upon by the EuroSpA Scientific Committee. Registry data managers clarified data availability and uploaded anonymized data through the secure Virtual Private Network pipelines to the EuroSpA server. Baseline characteristics, drug retention and response rates were investigated with non-parametric descriptive statistics. Kaplan-Meier estimation was used to investigate TNFi retention rates. Both crude and Lundex adjusted2 response rates were calculated for DAS28 remission (DAS28≤2.6) after 6, 12 and 24 months and ACR20/50/70 after 6 months.
Results:
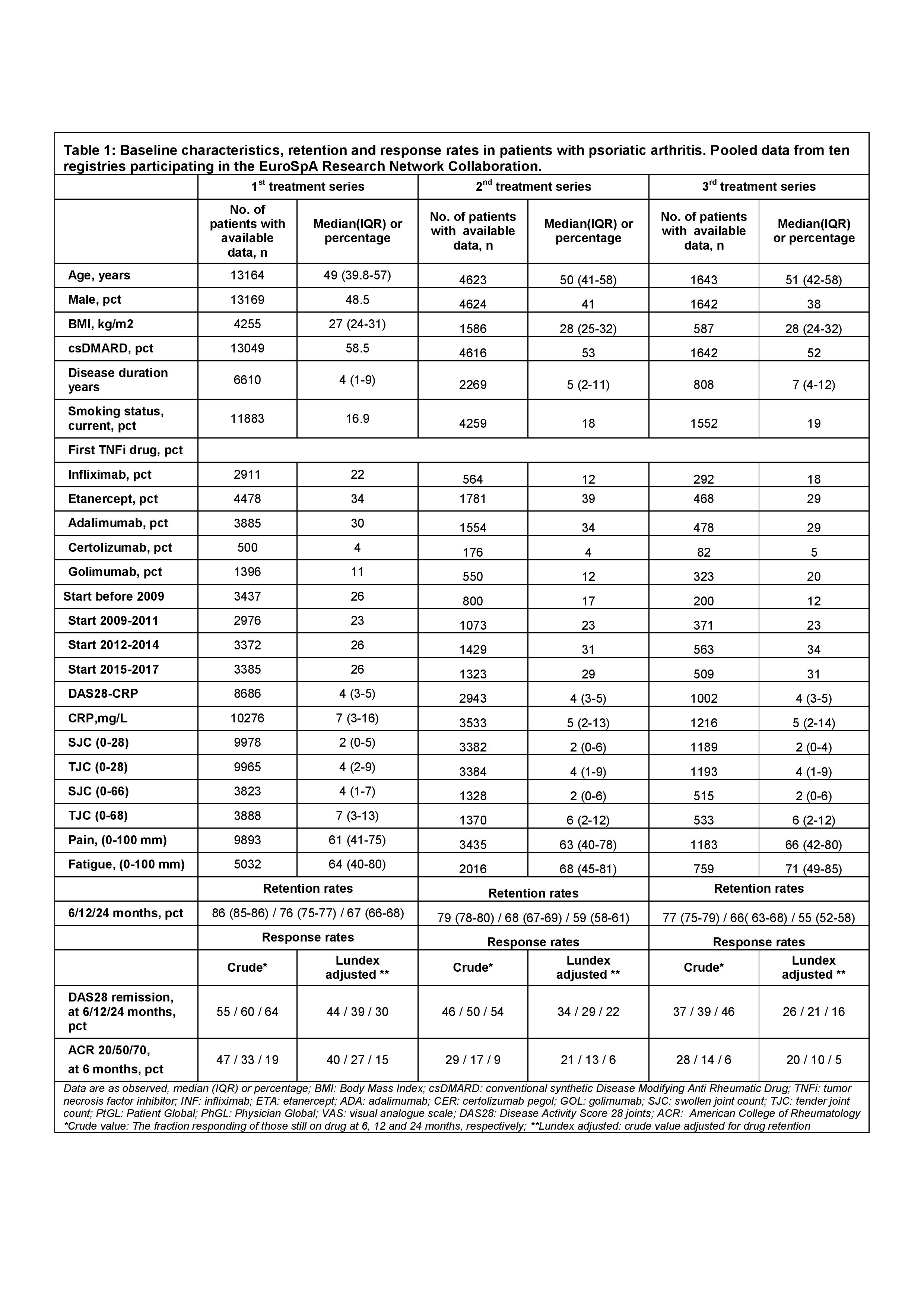
In May 2018, 10 of the 15 registries participating in EuroSpA had completed data upload to the EuroSpA server, including 13,170 patients with PsA. Baseline characteristics of the pooled population are shown in Table.
For the 1st TNFi, 6 and 24 months´ retention rates were 86% and 67%, respectively. Corresponding retention rates for the 2nd TNFi were 79% and 59%, and for the 3rd TNFi 77% and 55%, respectively (Table and Figure). For the 1st TNFi, 6 and 24 months Lundex adjusted DAS28 remission rates were 44% and 30%. Corresponding remission rates for the 2nd TNFi were 34% and 22%, and for the 3rd TNFi, 26% and 16%. For the 1st TNFi, 6 months Lundex adjusted ACR 20/50/70 response rates were 40%, 27% and 15%, respectively. Corresponding ACR 20/50/70 response rates for the 2nd TNFi were 21%, 13% and 6% and for the 3rd TNFi, 20%, 10% and 5%, respectively.
Conclusion:
These initial analyses demonstrate that the creation of a large European database of PsA patients treated in routine care based on a common data model is feasible, offering important opportunities for future research. In this pooled dataset from 10 European registries, we found decreasing retention rates and response rates with increasing number of previous TNFi.
References: 1)Ann Rheum Dis, 2017, suppl. 2, p.65
References: 2)Arthritis Rheum, 2006, 54(2), p.600-6.
To cite this abstract in AMA style:
Brahe CH, Ørnbjerg LM, Jacobsson L, Nissen MJ, Kristianslund EK, Santos MJ, Eklund K, Rotar Z, Gudbjornsson B, Onen F, Codreanu C, Lindström U, Gabay C, Kvien T, Barcelos A, Aaltonen K, Tomšič M, Love T, Can G, Ionescu R, Loft AG, Mann HF, Pavelka K, van de Sande M, van der Horst-Bruinsma I, Gomez-Reino JJ, Sánchez-Piedra C, Macfarlane GJ, Iannone F, Hyldstrup L, Krogh NS, Østergaard M, Hetland ML. Drug Retention and Response Rates of TNFi Treatment in 13,170 Patients with Psoriatic Arthritis Treated in Routine Care – Pooled Data from the Eurospa Research Network Collaboration [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/drug-retention-and-response-rates-of-tnfi-treatment-in-13170-patients-with-psoriatic-arthritis-treated-in-routine-care-pooled-data-from-the-eurospa-research-network-collaboration/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-retention-and-response-rates-of-tnfi-treatment-in-13170-patients-with-psoriatic-arthritis-treated-in-routine-care-pooled-data-from-the-eurospa-research-network-collaboration/