Session Information
Date: Sunday, October 26, 2025
Title: (0357–0386) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: IL-17A inhibitors (IL-17Ai) are considered recommended biologic therapies for PsA per current treatment guidelines1,2. Real-world evidence (RWE) studies on IL-17Ai drug persistence among patients with PsA are limited3. RWE data on long-term use of biologics can support more informed treatment strategies and improve patient outcomes4. Here, we analyse RWE data to assess the persistence of IL-17Ai therapy in patients with PsA.
Methods: This observational study included adult patients with a diagnosis of PsA enrolled in the CorEvitas PsA/SpA Registry. Primary and sensitivity analyses were conducted among IL-17Ai initiators, defined as patients who initiated ixekizumab or secukinumab as first use within the IL-17Ai mechanism of action (MOA) and had at least 1 follow-up registry visit. The primary outcome was drug persistence defined as time from treatment initiation of a specific MOA to discontinuation (DC) of that MOA. For sensitivity analysis, persistence was redefined as continuous use of initiated therapy without DC, with cycling to another IL-17Ai therapy considered as DC. Each outcome was censored at the last available study visit or 12 months (M12), whichever occurred first. Drug persistence at M12, with corresponding 95% confidence intervals (CI), was estimated using the Kaplan-Meier method. Unadjusted Cox proportional hazards models were used to estimate hazard ratios (HR) and corresponding 95% CI between patient characteristics and DC.
Results: A total of 902 patients with PsA initiated a first IL-17Ai. Demographic, disease and treatment characteristics at index are presented in Table 1. Secukinumab was initiated by the majority (74.9%) of the IL-17Ai cohort, with ixekizumab accounting for 25.1%. (Table 1). For the primary outcome, proportion persistent with a specific MOA at M12 was estimated to be 62.4% (95% CI: 59.2, 65.8) (Figure 1). Median persistence was not reached at M12. Persistence was numerically lower in the sensitivity analysis (55.7% [95% CI: 52.4, 59.2]). Unadjusted univariate analysis showed that numerically some factors including female sex, history of fibromyalgia, depression, current smoker, >2 comorbidities, axial involvement among others had an increased risk of IL-17Ai DC (HR >1) (Table 2), with similar results in the sensitivity analysis.
Conclusion: Findings from this observational study of patients with PsA demonstrate the persistence of IL-17Ai, as a class, in a real-world setting. Persistence estimates at M12 align with patient retention rates observed in clinical trials5 and previous registry-based studies6 for IL-17Ai cohorts. Numerous factors demonstrated a higher risk of decreased persistence, with CIs not suggesting statistical significance. This underscores the effect that disease burden in patients with PsA can have on treatment outcomes, affecting the persistence of treatments7.ReferencesCoates, LC, Nat Rev Rheumatol, 18 (2022) 465Gossec, L, Ann Rheum Dis, 83 (2024) 706Joven, B, Adv Ther, 40 (2023) 5415Rohekar, S, ACR Open Rheumatol, 6 (2024) 440VD-Heijde, D, J Rheumatol, 45 (2018) 367López-Medina, C, Ann Rheum Dis, 83 (2024) 1494Campanholo, CB, J Rheumatol, 50 (2023) 426
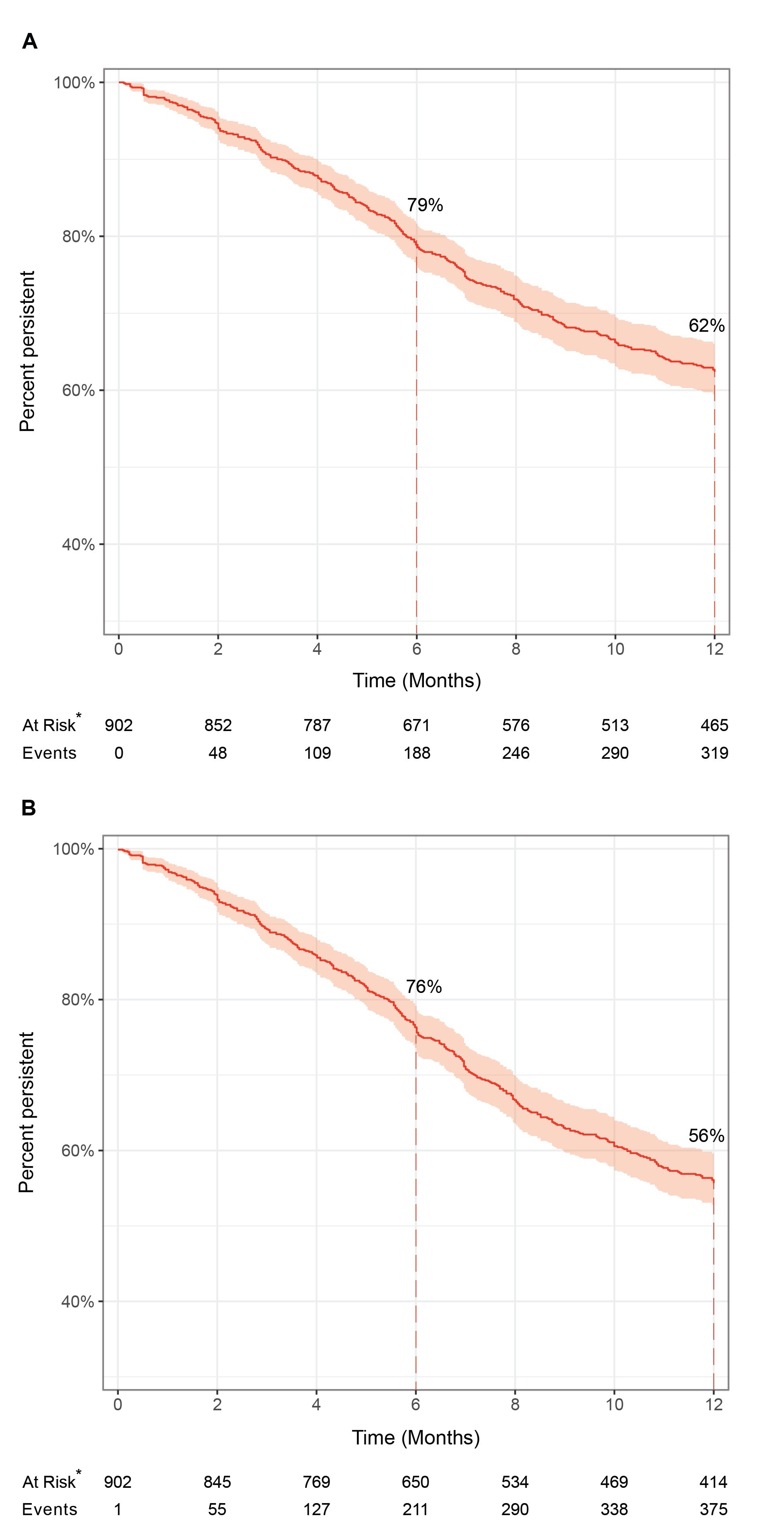 Figure 1: Kaplan-Meier plots of drug persistence among IL-17Ai initiators for the (A) primary analysis and (B) sensitivity analysis
Figure 1: Kaplan-Meier plots of drug persistence among IL-17Ai initiators for the (A) primary analysis and (B) sensitivity analysis
*This does not represent the outcome of interest, rather the number of patients remaining at risk for the outcome at each timepoint.
The pink shaded area represents the 95% confidence interval.
To cite this abstract in AMA style:
Mease P, Middaugh N, Marchese M, Peterson S, Ertugrul Vardar B, Burge R, Bello N, Ngantcha M, Merola J, Bessette L, Ogdie A. Drug Persistence of IL-17A Inhibitors Among Patients with PsA: Real-World Data from the CorEvitas PsA/SpA Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/drug-persistence-of-il-17a-inhibitors-among-patients-with-psa-real-world-data-from-the-corevitas-psa-spa-registry/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-persistence-of-il-17a-inhibitors-among-patients-with-psa-real-world-data-from-the-corevitas-psa-spa-registry/

.jpg)
.jpg)