Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: IgA vasculitis (IgAV) is an immune complex small-vessel vasculitis, with IgA1-dominant immune deposits. Drug-induced IgAV were rarely reported in the literature, but no systematic analysis was done so far. We used a novel approach combining the analysis of two pharmacovigilance (PV) databases to identify the main drugs associated with drug-induced IgA.
Methods: We used VigiBase, the WHO global individual case safety reports database, and the FPVD (French PV Database), to extract drug-induced IgAV using MedDRA terms compatible with the diagnosis of IgAV, between database inception and Jan 2019. Detailed narratives of cases from BNPV were analyzed by two investigators and then categorized into possible or definitive IgAV based on predefined criteria. Demographic and clinical data on IgAV, drugs class and chronological relationship with IgAV were described. Using VigiBase data, we evaluated the association between IgAV and drugs by conducting disproportionality analyses (case-noncase method) calculating reporting odds ratios (RORs). Significance was reached if RORs were >1.
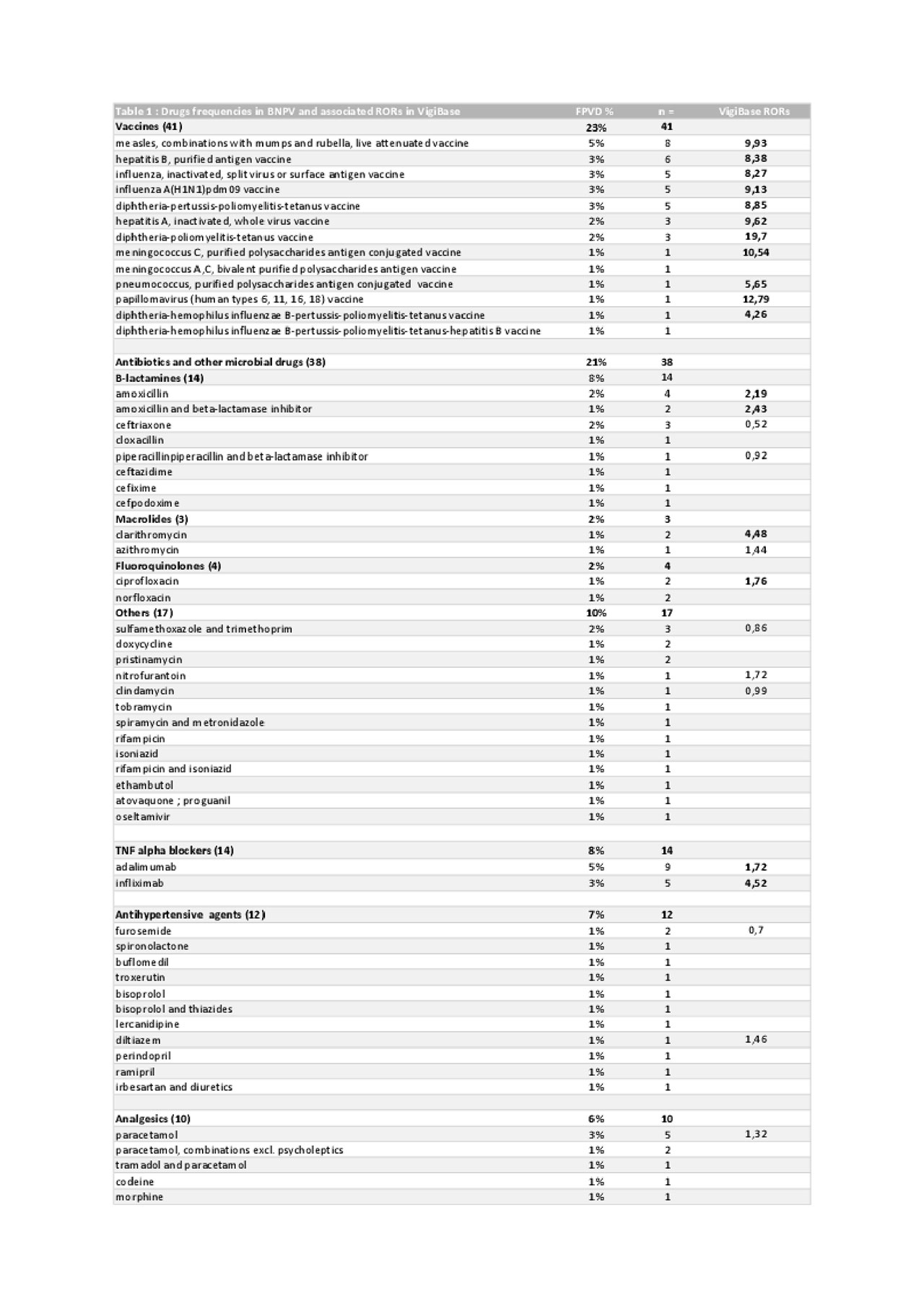
Results: From the 466 cases extracted from the FPVD database, we finally included 113 definitive or possible IgAV cases (for which 177 drugs were suspected as potential triggers); and among 18,518,924 individual case safety reports from VigiBase, we included 1242 cases (associated with 40 drugs with significant RORs).
FPVD population was mainly male (58%), with a median age of 28 years. Main IgAV clinical characteristics were purpura in 100%, joint involvement in 55%, gastrointestinal involvement in 36% and renal involvement in 33%. No vasculitis-specific treatment was required in 67%, whereas 32% of patients received glucocorticoids, 3% colchicine, and 1% cyclophosphamide. Outcome was favorable in 60% of patients, but 16% had sequelae, most frequently mild to moderate (only 2 patients had chronic renal failure). No death was reported during follow up.
The drugs associated with the highest number of drug-induced IgAV were vaccines (23% of all treatments), antibiotics and others antimicrobial drugs (21%), and tumor necrosis factor (TNF)-α blockers (8%). Median interval between initiation of vaccines, antibiotics/antimicrobial drugs and TNF-α blockers and IgAV onset were 11 days, 10 days and 26.5 months, respectively.
Frequencies of drugs identified within the FPVD and their associated RORs calculated within VigiBase are summarized in the Table.
Conclusion: Our study enables the identification of the main drugs associated with drug-induced IgAV using a combined approach of the detailed narratives from the FPVD and the large international Vigibase. Main suspected drugs were vaccines, antibiotics and TNF-α blockers in both databases. This list of suspected drugs may prove useful to physicians when confronted with potential IgAV cases.
To cite this abstract in AMA style:
RASMUSSEN C, TISSEYRE M, GARON-CZMIL J, ATZENHOFFER M, Guillevin L, CHOUCHANA L, Terrier B. Drug-induced IgA Vasculitis: Data from the French Pharmacovigilance Network and the WHO VigiBase [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/drug-induced-iga-vasculitis-data-from-the-french-pharmacovigilance-network-and-the-who-vigibase/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-induced-iga-vasculitis-data-from-the-french-pharmacovigilance-network-and-the-who-vigibase/