Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Peripheral spondyloarthritis (pSpA) is a neglected clinical entity with few therapeutic or clinical management trials available to date. In the CRESPA-trial (1) very early pSpA patients, all fulfilling the Assessment of SpondyloArthrtis international Society (ASAS) classification criteria for pSpA and having a symptom duration ≤12 weeks, were randomized to golimumab (n=40) or placebo (n=20). Placebo-treated patients were switched to golimumab at week 24 if no sustained clinical remission was achieved (primary end point) (1). In patients achieving sustained clinical remission, treatment was stopped to assess the possibility of drug-free remission (2). In those not achieving the primary endpoint at week 48, treatment with golimumab was continued (2). The objective of this study was to determine the percentage of patients achieving a status of clinical remission after a follow-up of 10 years.
Methods: In this 10-year follow-up study patients that participated in the CRESPA-trial were evaluated through clinical examination assessing arthritis, enthesitis and dactylitis. All evaluations were done between March 2024 and September 2024. Clinical remission was defined as the absence of arthritis, enthesitis or dactylitis. A secondary aim was to identify baseline differences between patients currently in remission and those not in remission.
Results: As reported previously, sustained clinical remission was achieved in 82% (49 of 60) of patients after an induction regimen, 15 placebo treated patients needed golimumab (1) (figure 1). 1 patient was excluded due to progression to seronegative rheumatoid arthritis. Of those 60 patients originally included in the CRESPA study, we were able to evaluate 49 patients within an average of 10.5 years (SD 0.87 years) of follow-up. Clinical remission was observed in 82% (40 of 49) patients, with 37.5% (15 of 40) of them in drug-free remission (figure 2). 6 patients were on MTX monotherapy and 28 patients on bDMARD (57% monotherapy, 43% in combination with MTX). 21 patients still use golimumab, with no unsuspected serious adverse effects reported. Of the 9 patients not in clinical remission after 10 years of follow-up, 7 experienced enthesitis, 1 arthritis and 1 both enthesitis and arthritis. There were no baseline differences regarding sex, age, HLA B27-positivity, psoriasis, or initial treatment allocation between patients in clinical remission and those not in clinical remission. However, drug-free remission was significantly less common in patients with psoriasis compared to those without (OR = 0.11, 95% CI: 0.02–0.55, p = 0.005).
Conclusion: After 10 year follow-up, the vast majority of patients are in clinical remission with 37.5% of them even in drug-free remission. The presence of psoriasis at baseline was associated with a lower likelihood of achieving drug-free remission. References: 1) Carron P, Varkas G, Cypers H, et al. Anti-TNF-induced remission in very early peripheral spondyloarthritis: the CRESPA study. Ann Rheum Dis. 2017;76(8):1389–95. 2) Carron P, Varkas G, Renson T, et al. High Rate of Drug Free Remission After Induction Therapy With Golimumab in Early Peripheral Spondyloarthritis. Arthritis Rheumatol. 2018;70(11):1769-77.
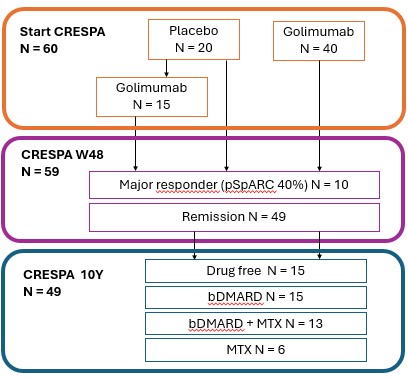 Medication use during CRESPA trial and after 10 year follow-up
Medication use during CRESPA trial and after 10 year follow-up
.jpg) Remission at the end of the CRESPA trial and after 10 year follow-up
Remission at the end of the CRESPA trial and after 10 year follow-up
To cite this abstract in AMA style:
Damen c, Carron P, De Craemer A, Van den Bosch F, Elewaut D. Drug-Free Remission in Early Peripheral Spondyloarthritis: 10-year follow-up from the CRESPA-trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/drug-free-remission-in-early-peripheral-spondyloarthritis-10-year-follow-up-from-the-crespa-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-free-remission-in-early-peripheral-spondyloarthritis-10-year-follow-up-from-the-crespa-trial/
