Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Biosimilars hold promise for reducing pharmaceutical expenditures, however uptake has lagged. We analyzed the variability in new biosimilar starts and switching from bio-originator infliximab to its biosimilar counterpart across rheumatology practices nationally.
Methods: Data came from RISE, a national registry with electronic health records from over 1100 US rheumatologists. We included 37,560 patients age > 18 years administered infliximab (bio-originator or biosimilar) between April 2016 to September 2022. We tested for differences in ever use of biosimilar infliximab by demographic characteristics, socioeconomic status and diagnosis using logistic regression. We used generalized estimating equations to assess the adjusted effect of insurance and year of initiation on new biosimilar starts. We analyzed variation in biosimilar switching by insurance, date of switch, and practice. We assessed the portion of the variance in switching and new starts on a biosimilar at the practice level using the intraclass correlation coefficient.
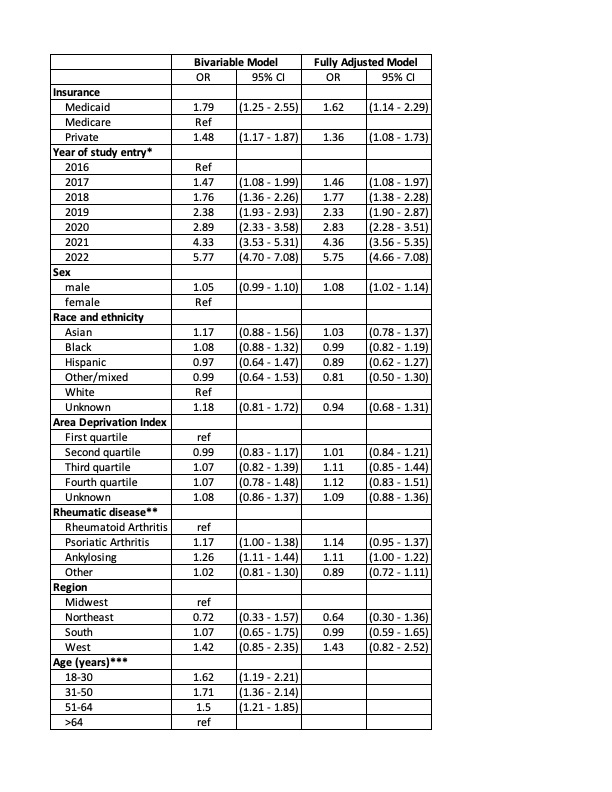
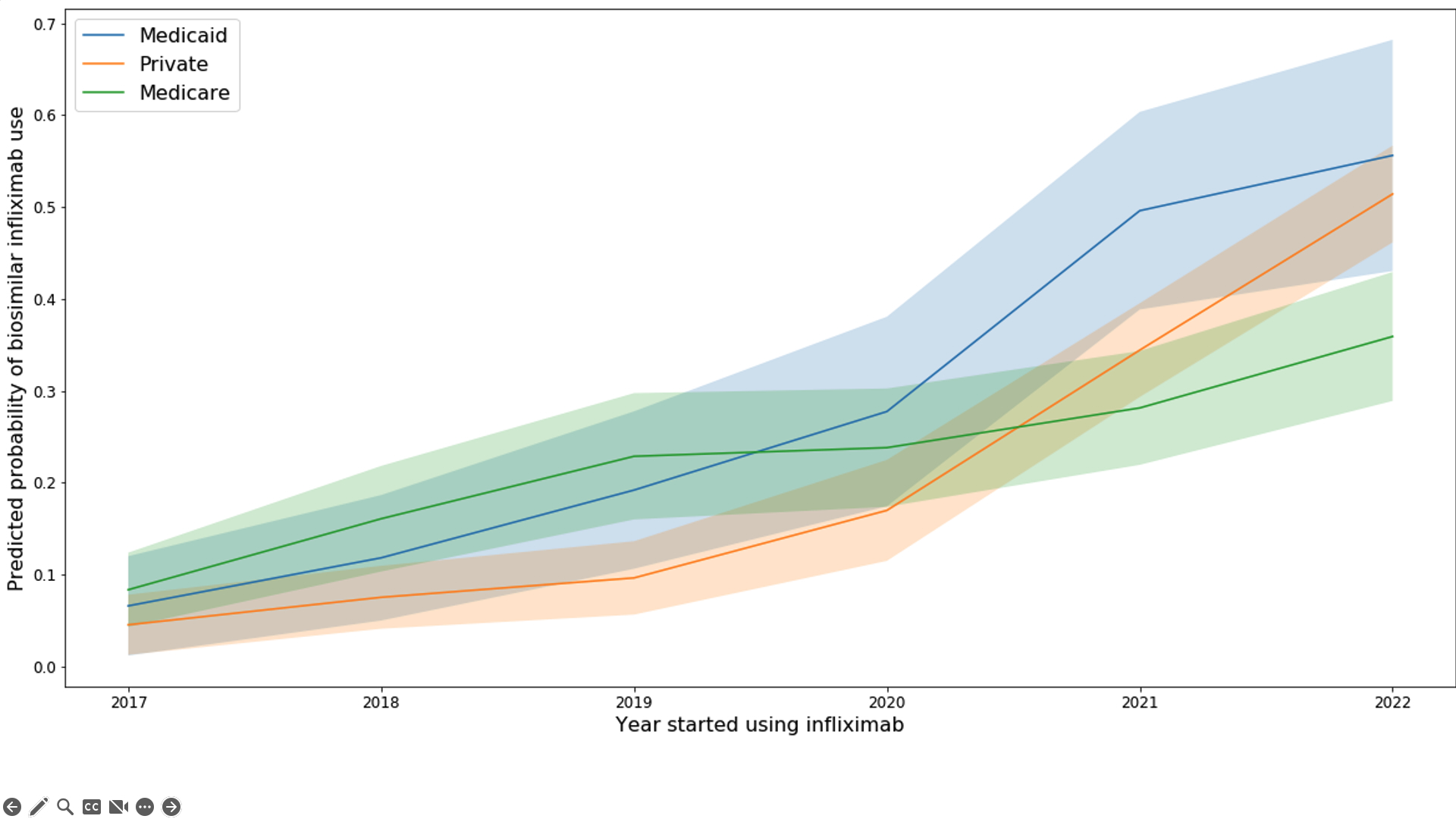
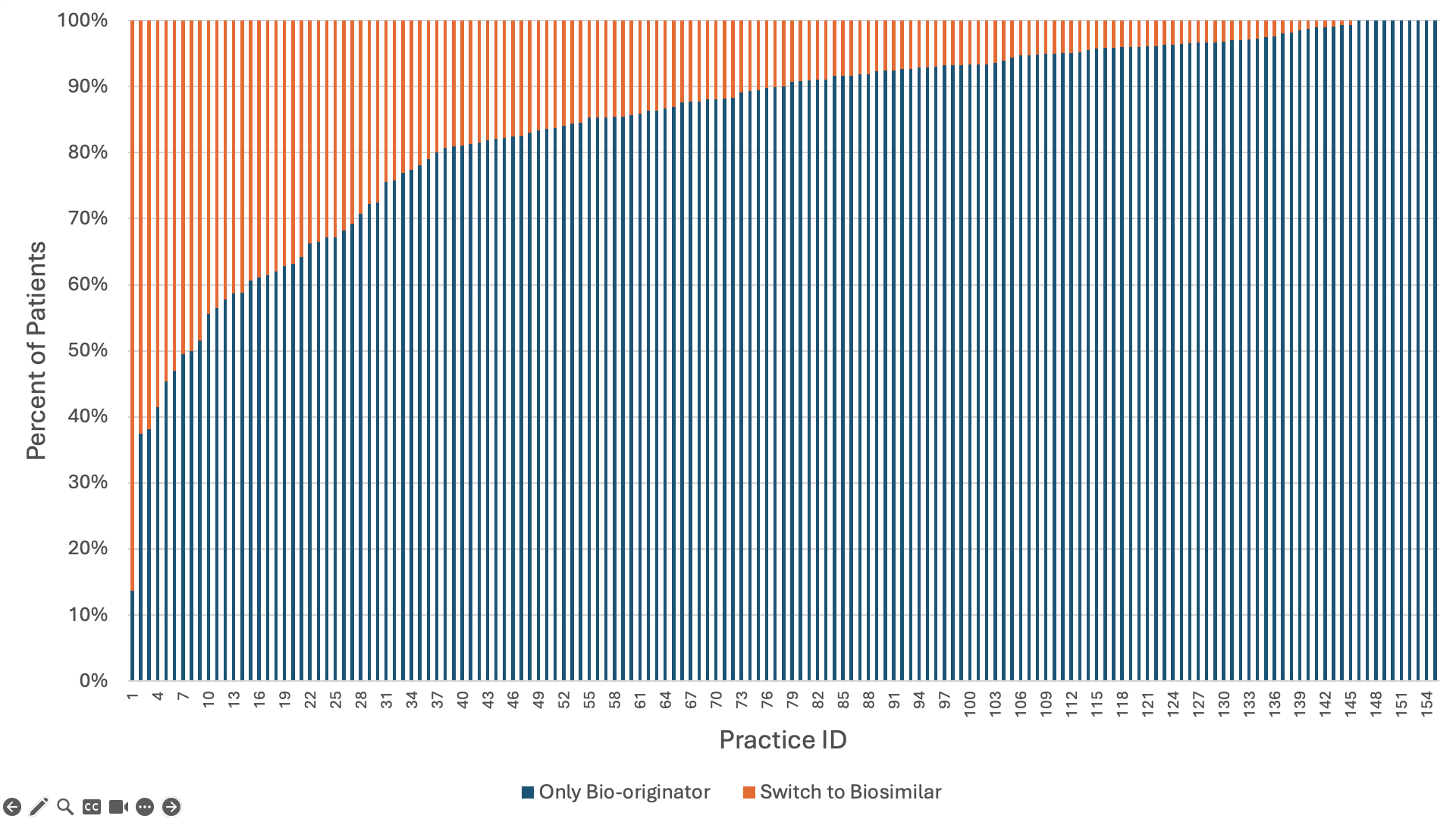
Results: 8,196 (21.8%) infliximab users ever used a biosimilar. 52% of patients had Medicare, 43% had private insurance, and 5% had Medicaid. Overall, 52% were 65 years or older, 70% were female, 72% were non-Hispanic white. Most patients had rheumatoid arthritis (58%), 11% had psoriatic arthritis and 10% had ankylosing spondylitis. In our final model, the strongest predictors of ever receiving biosimilar infliximab were insurance (Medicaid OR = 1.62, 95%CI 1.14-2.29; Private OR = 1.36, 95%CI 1.08-1.73 compared to Medicare) and the year the patient entered the study (ranging from OR = 1.46, 95%CI 1.08-1.97 in 2017 to OR = 5.75, 95%CI 4.66-7.08 in 2022 compared to 2016) (Table 1). Overall, 21.6% of new starts were on a biosimilar. In 2022, new starts on a biosimilar occurred more frequently in Medicaid (55%) and private insurance (51%) compared to Medicare (37%) (Figure 1). Overall, 11.6% of prevalent bio-originator infliximab users switched to a biosimilar, and switching was lowest among Medicare beneficiaries (7% vs. 14.2% in Medicaid and 16.9% among privately insured). Switching became more likely over time (63.1% of switches occurred in 2021 or 2022). Across practices, the median proportion of patients switched from bio-originator to biosimilar was 10% (IQR=4.2-19%) but 37 practices switched >20% of their patients (Figure 2). Practice-level differences explained 37% of the variation among new biosimilar starts and 34% of the variation among those switching to a biosimilar.
Conclusion: Our findings underscore two critical areas for enhancing biosimilar infliximab use: 1) increasing switching among prevalent users, since using a biosimilar was significantly more common among new users than prevalent users, and 2) increasing uptake among Medicare beneficiaries initiating treatment. Significant variation in uptake across practices also suggests that local switching policies are likely key drivers of uptake. Given that only 21.8% of patients ever used a biosimilar, promoting switching is crucial to increase biosimilar competition and potentially reduce prices.
*Data used in this analysis is left censored. Users with a first year of use in 2016 are a mix of prevalent and incident users.
**Patients were only assigned to one diagnosis category: PSA>AS>RA
***Age was not controlled for in multivariable models as age > 64 is highly correlated with Medicare insurance.
Disclaimer: This data was supported by the ACR’s RISE Registry. However, the views expressed represent those of the authors, not necessarily those of the ACR.
To cite this abstract in AMA style:
Roberts E, Bansback N, Tseng C, Shiboski S, Li J, Schmajuk G, Yazdany J. Drivers of Infliximab Biosimilar Uptake: A Comparative Analysis of New Biosimilar Initiations versus Switching in a National Rheumatology Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/drivers-of-infliximab-biosimilar-uptake-a-comparative-analysis-of-new-biosimilar-initiations-versus-switching-in-a-national-rheumatology-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drivers-of-infliximab-biosimilar-uptake-a-comparative-analysis-of-new-biosimilar-initiations-versus-switching-in-a-national-rheumatology-registry/