Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The potential for reactivation of tuberculosis (TB) in patients treated with anti-TNF or other immunosuppressive agents has been well described. Interferon-gamma release assays (IGRA) have improved latent TB infection (LTBI) detection. Evidence is accumulating that shows a negligible yield for periodic testing in U.S. born rheumatology patients. Does this evidence apply to patients born in TB endemic countries now living in the U.S.? Incident active TB disease in the United States disproportionately affects people born in countries with endemic TB.
Purpose: To determine the yield of serial TB screening using an IGRA (Quantiferon Gold-QFT) in a United States rheumatology clinic with a majority (80%) foreign born population.
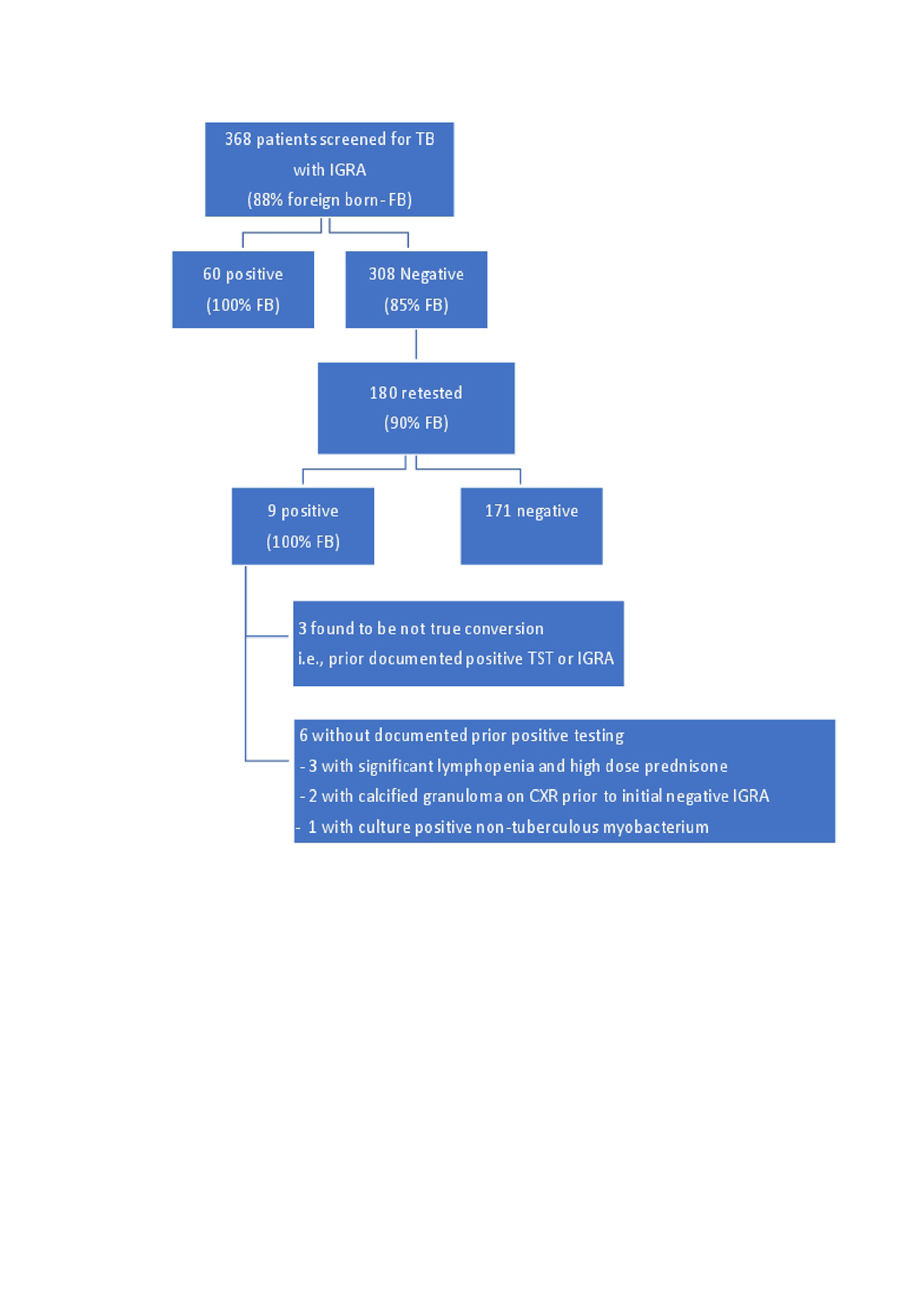
Methods: We sought to determine the utility of serial TB screening in this population using retrospective cross- sectional study design. Between January 2013 and December 2018, a total of 368 patients received TB screening using IGRA. Positive results were classified as initial positive or as a conversion if there was evidence of prior negative test. Records showing conversion were reviewed further for evidence of prior positive result outside of our institution and reclassified as not true conversions accordingly.
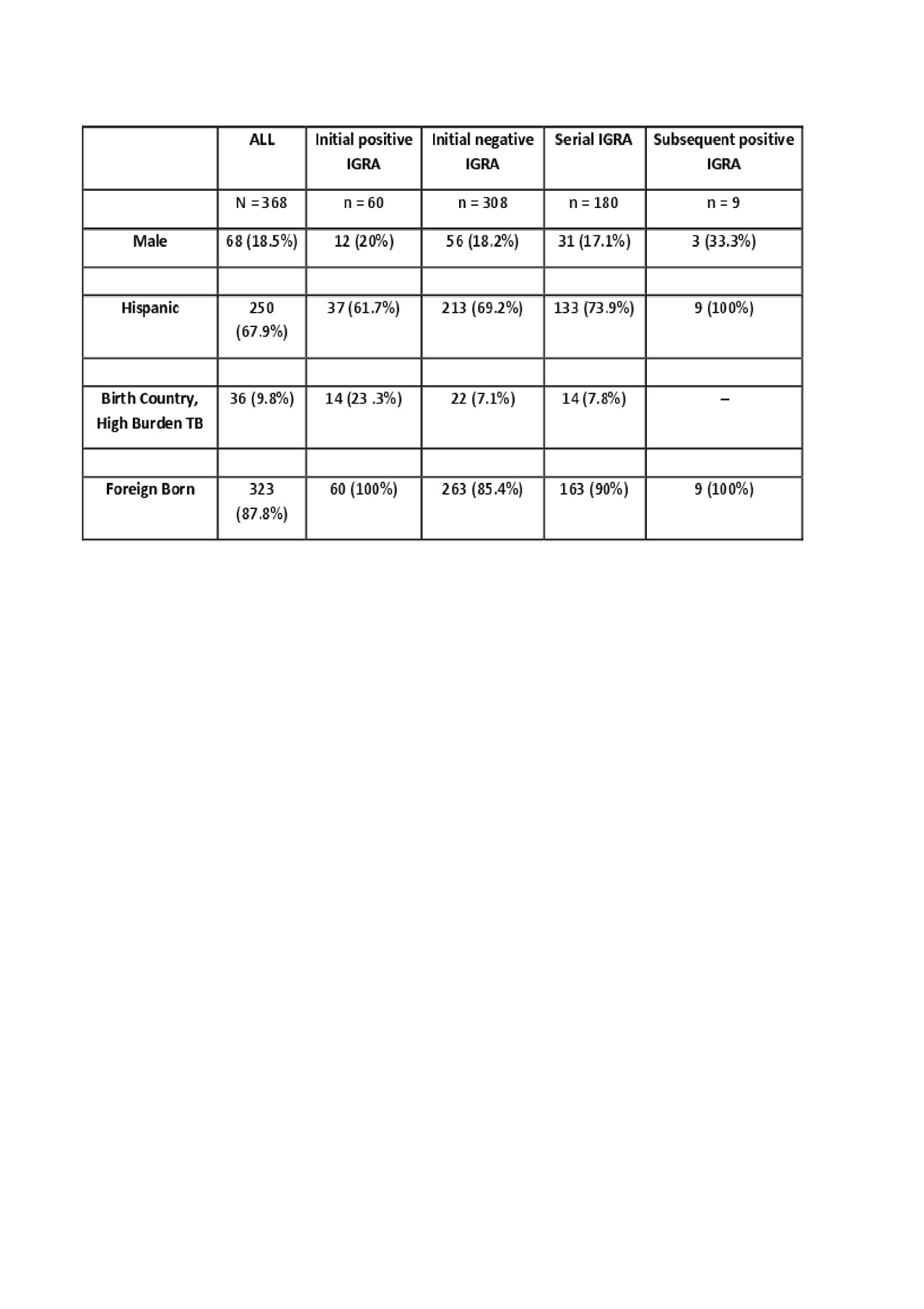
Results: During the study period, 368 subjects received TB screening using an IGRA. Of those, 60 initially screened positive (16%). The initially positive patients were 100% foreign born and twice as likely than the general clinic population to have been born in a country WHO designated as high burden for TB. 308 patients were initially screened negative. Of those, 180 received serial testing with subsequent testing at least 6 months after the initial test. Nine of those patients later tested positive (5%); all were foreign born. Of these nine, further review revealed three were not true conversions. Of the six who appeared to have a true conversion, three were noted to have had significant lymphopenia (180-660 absolute) and were receiving high doses of prednisone (25-60 mg/day) at the time of their initial negative IGRA. Two other patients were noted to have had calcified granuloma on chest x-ray at the initial negative IGRA. The sixth patient was found to have non-tuberculous mycobacterium and was treated. There were no cases of active TB during the study period. No patients born in the United States tested positive at initial or subsequent testing.
Conclusion: Our study demonstrates utility in serial TB screening with an IGRA in a patient population with birth in TB endemic countries, as well as periodic travel to such regions. In our patient population the yield was greatest in patients from countries with a high burden of TB. History is vital when ordering an IGRA, particularly at the initial testing. Prior positive TB skin test, prior evaluation at the local health department, prior exposure to individuals with TB, or prior TB infection, exposure, and/or treatment should be a regular component of history-taking in clinics responsible for managing immunosuppressive agents. It is important to keep in mind the risk of occult LTBI in patients who are immunosuppressed and born in TB endemic countries despite a negative IGRA result.
To cite this abstract in AMA style:
Fike A, Ruiz-Perdomo Y, Katz J. Double Trouble: Utility of Serial TB Screening in a Rheumatology Clinic Serving a Foreign-Born Population [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/double-trouble-utility-of-serial-tb-screening-in-a-rheumatology-clinic-serving-a-foreign-born-population/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/double-trouble-utility-of-serial-tb-screening-in-a-rheumatology-clinic-serving-a-foreign-born-population/