Background/Purpose:
Denosumab is a fully human monoclonal antibody (IgG2 subclass) that binds specifically to RANKL. It inhibits osteoclast-induced bone resorption by preventing the binding of RANKL to RANK, thereby inhibiting progression of marginal bone erosion at the joints where inflammatory cytokines are highly expressed, stopping structural joint damage. In this study, we evaluated the dose-frequency of denosumab for safety and efficacy in Japanese patients with RA.
Methods:
DRIVE Study (AMG162-D-J201) was a placebo-controlled, 4-arm randomized (1:1:1:1), double-blind, parallel-group study in patients receiving methotrexate treatment. Denosumab 60 mg (every 6 months (Q6M), 3 months (Q3M), or 2 months (Q2M)) or placebo was administered subcutaneously for 12 months. The primary endpoint was change in the bone erosion score assessed by the modified Sharp-van der Heijde method from baseline to 12 months. One-tailed Shirley-Williams’s test were performed and differences were considered significant when p<0.025.
Results:
Among 350 patients enrolled, 346 (placebo, n=88; Q6M, n=86; Q3M, n=85; Q2M, n=87) were treated with the study drugs (Table 1), and 35 (placebo n=6; Q6M, n=8; Q3M, n=13; Q2M, n=8) were discontinued.
Table 1. Baseline demographics and characteristics of Japanese patients with RA.
|
Characteristic |
Placebo |
Denosumab 60 mg |
||
|
Q6M |
Q3M |
Q2M |
||
|
Female |
76 (86.4) |
65 (76.5 |
59 (72.0) |
66 (77.6) |
|
Age (years) |
57.0 ± 10.6 |
54.4 ± 10.6 |
52.0 ± 11.7 |
54.6 ± 10.5 |
|
Disease duration (years) |
2.3 ± 1.3 |
2.2 ± 1.3 |
2.3 ± 1.3 |
2.3 ± 1.4 |
|
Rheumatoid factor positive |
60 (68.2) |
59 (69.4) |
56 (68.3) |
57 (67.1) |
|
MTX dose (mg) |
7.6 ± 1.8 |
8.0 ± 2.0 |
8.4 ± 2.2 |
8.3 ± 2.0 |
|
DAS28-CRP |
3.5 ± 0.9 |
3.2 ± 0.9 |
3.3 ± 0.9 |
3.3 ± 0.8 |
Data presented are Mean ± SD or n (%).
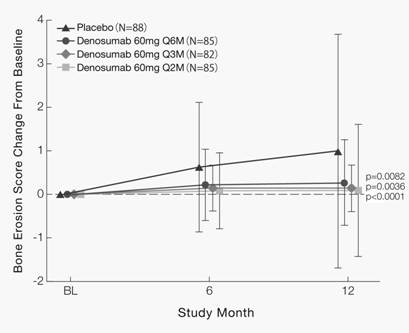
Changes in the bone erosion score (deltaERO) at Month 12 were 0.99 ± 2.69 in placebo, 0.27 ± 0.98 in Q6M (Compared with placebo, p=0.0082), 0.14 ± 0.53 in Q3M (p=0.0036), and 0.09 ± 1.52 in Q2M (p<0.0001) (Figure 1). Percentages of patients without increase in ERO (≤0) at Month 12 were 62.5% in placebo, 78.8% in Q6M (p=0.0173), 80.5% in Q3M (p=0.0099), and 83.5% in Q2M (p=0.0019).
Figure 1. Mean (± SD) Bone Erosion Score Change From Baseline (Full Analysis Set)
No significant differences in the types and the overall incidence of adverse events (AEs) were observed among denosumab-treated groups and the placebo group. No hypocalcemia, osteonecrosis of the jaw, or atypical femoral fracture were observed.
Conclusion:
Greater suppression of the bone erosion from baseline to 12 months and the less erosive progression were observed after denosumab treatment in all doses compared with placebo, with lower progression rates in groups with shorter dosing interval. Denosumab was well tolerated in Japanese patients with RA and shortening the dosage interval did not increase AE incidence.
Disclosure:
T. Takeuchi,
Abott Japan, Astellas Pharma, Bristol-Myers, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Mitsubishi Tanabe Pharma, Pfizer, Sanofi-aventis, Santen Pharmaceutical., Takeda Pharmaceutical, Teijin Pharma, AbbVie, Asahi Kasei Pharma, Taisho Toyama Pharma,
2,
Abott Japan, Bristol-Myers, Chugai Pharmaceutical, Eisai, Janssen Pharmaceutical, Mitsubishi Tanabe Pharma, Pfizer Japan, Takeda Pharmaceutical, Astellas Pharma, Daiichi Sankyo, Astra Zeneca, Eli-Lilly Japan, Novartis Pharma, Asahi Kasei Pharma, AbbVie,
5;
Y. Tanaka,
Bristol-Myers, Mitsubishi Tanabe Pharma, AbbVie, MSD, Chugai Pharmaceutical, Astellas Pharma, Daiichi Sankyo,
2,
Mitsubishi Tanabe Pharma, Eisai, Chugai Pharmaceutical, Abott Japan, Astellas Pharma, Daiichi Sankyo, AbbVie, Janssen Pharmaceutical, Pfizer Japan, Takeda Pharmaceutical, Astra Zeneca, Eli Lilly Japan, GlaxoSmithKline, Quintiles, MSD, Asahi Kasei Pharma,
5;
N. Ishiguro,
Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Astellas Pharma, Chugai Pharmaceutical, Abott Japan, Bristol-Myers, Eisai, Janssen Pharmaceutical, Kaken Pharmaceutical, Pfizer Japan, Taisho Toyama Pharmaceutical, Otsuka Pharmaceutical, Daiichi Sankyo,
5;
H. Yamanaka,
Abott Japan, Bristol-Myers, Chugai Pharmaceutical, Eisai, Mitsubishi Tanabe Pharma, Takeda Pharmaceutical, Pfizer Japan,
5;
T. Yoneda,
Daiichi Sankyo,
5;
H. K. Genant,
Amgen, Bristol-Myers Squibb, Eli Lilly & Co., Genentech, GlaxoSmithKline, Merck & Co., Novartis Pharmaceuticals, Pfizer, Roche Pharmaceutical, ONO Pharma USA, Servier, and Synarc,
5;
D. van der Heijde,
AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Centocor Biotech, Chugai Pharmaceutical, Eli Lilly & Co., GlaxoSmithKline, Imaging Rheumatology, Merck & Co., Novartis Pharmaceuticals, Pfizer, Roche Pharmaceutical, Sanofi-Aventis, Schering-Ploug,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dose-response-effects-of-denosumab-a-novel-subcutaneous-rankl-inhibitor-on-the-progression-of-bone-erosion-in-japanese-patients-with-rheumatoid-arthritis-treated-with-methotrexate-results-of-phase/