Session Information
Date: Tuesday, November 15, 2016
Title: Rheumatoid Arthritis – Clinical Aspects IV: Managing Patients in Remission
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Dose tapering in patients in remission has previously proven promising in randomized controlled trials. However, systematic implementation in clinical practice is lacking. A new guideline in the Capital Region of Denmark required that RA patients in sustained remission on biological therapy must attempt dose reduction according to a predefined algorithm. We aimed to 1) report the 1-year results of the implementation of the guideline and 2) investigate potential clinical baseline predictors of flare during the 1st year.
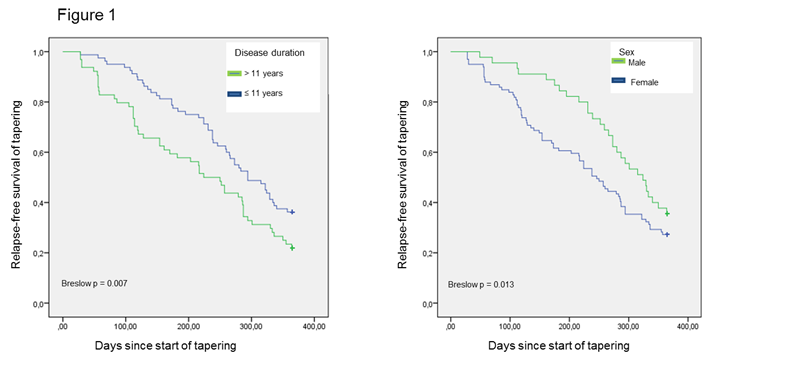
Methods: Patients with disease activity score (DAS28, 4 variables, CRP-based) ≤2.6 for ≥1 year and no radiographic progression the previous year were included. According to the algorithm, dosing of biological drug was to be reduced to 2/3 of standard dose at baseline, to ½ of standard dose after 4 months, and discontinued after 8 months. Patients who flared stopped tapering and were escalated to the previous dose to regain remission. Flare was defined as 1) DAS28≥2.6 AND DDAS28≥1.2 since baseline, or 2) erosive progression based on X-ray and/or magnetic resonance imaging (MRI). The relapse-free time since start of tapering stratified by gender and by median disease duration (11 years) was presented as Kaplan-Meier curves (time to flare). Breslow test was applied to test for significant difference between men and women and disease duration.
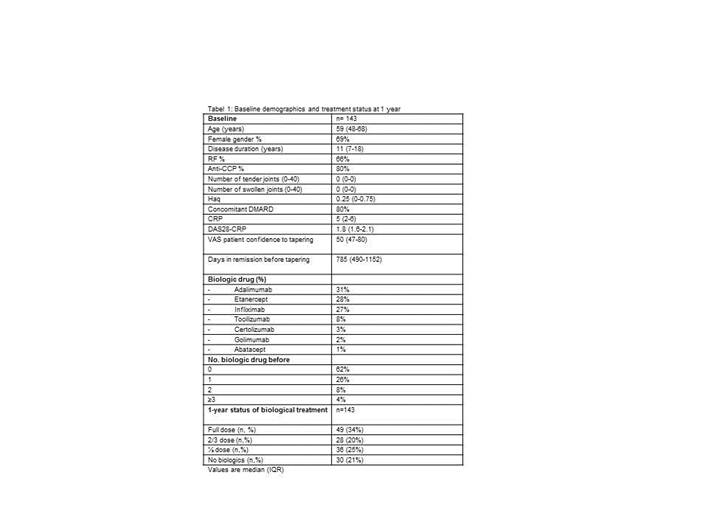
Results: A total of 143 patients from 5 departments of rheumatology were included as part of the implementation of the guideline. Baseline characteristics and medication are shown in table 1. During the 1st year, 101 (71%) patients flared and stopped tapering. All patients who flared were re-escalated and regained remission. At 1 year, 49 (34%) were on full dose, 28 (20%) patients were on 2/3 dose, 36 (25%) on half dose, and 30 (21%) had discontinued biological treatment. Median time to flare was 273 days (IQR: 247-299). According to figure 1 female sex and longer disease duration were associated with an increased risk of flare.
Conclusion: One year after initiating dose tapering in RA patients in remission in routine clinical practice, 66% of patients had been able to reduce the dose. Flares during tapering occurred in >70%, but were reversed by re-escalating patients to the previous dose. Female sex and longer disease duration were associated with increased risk of flare.
To cite this abstract in AMA style:
Brahe CH, Krabbe S, Østergaard M, Rogind H, Jensen HS, Hansen A, Nørregaard J, Jacobsen S, Terslev L, Huynh TK, Jensen DV, Manilo N, Asmussen KH, Brown-Frandsen P, Boesen M, Rastiemadabadi Z, Glinatsi D, Morsel-Carlsen L, Møller JM, Krogh NS, Lund Hetland M. Dose Reduction or Discontinuation of Biological Therapy in Patients with Rheumatoid Arthritis in Remission – 1-Year Results of a Guideline-Directed Longitudinal Cohort Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/dose-reduction-or-discontinuation-of-biological-therapy-in-patients-with-rheumatoid-arthritis-in-remission-1-year-results-of-a-guideline-directed-longitudinal-cohort-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dose-reduction-or-discontinuation-of-biological-therapy-in-patients-with-rheumatoid-arthritis-in-remission-1-year-results-of-a-guideline-directed-longitudinal-cohort-study/