Session Information
Date: Monday, November 18, 2024
Title: SLE – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: 2023 EULAR recommendations for the management of SLE open up the option for early treatment initiation with biologics without the requirement to fail immunosuppressants/DMARDs in patients who did not respond adequately to antimalarials alone or in combination with glucocorticoids (GC).1 There are limited data demonstrating clinical benefits of using biologics in immunosuppressant-naïve patients with SLE treated with either antimalarials, oral GCs, or the combination.
Objective
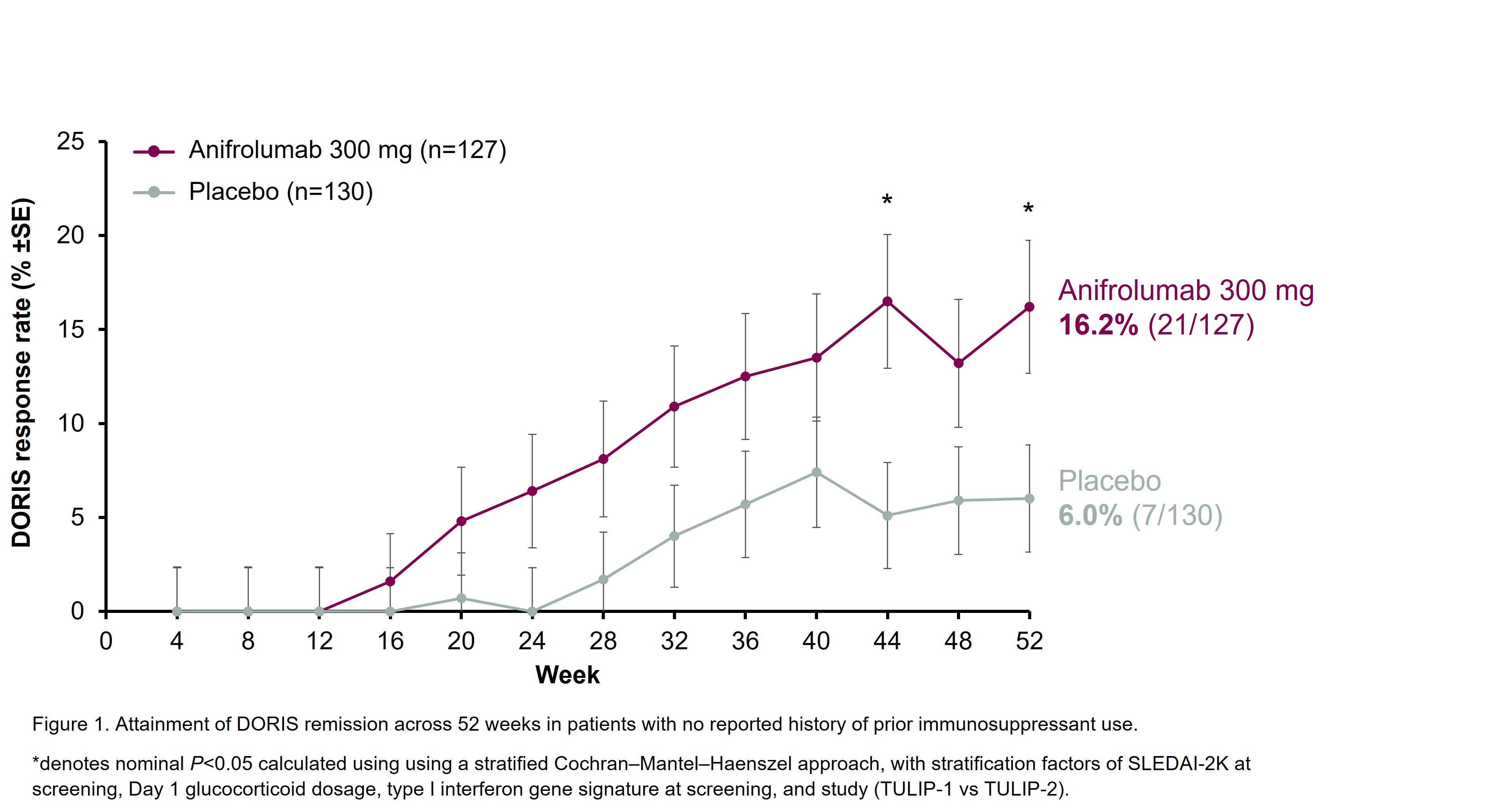
To investigate DORIS (Definition of Remission in SLE) remission attainment in patients with moderate to severe SLE treated with intravenous anifrolumab 300 mg or placebo in the TULIP-1 (NCT02446912)2 or TULIP-2 (NCT02446899)3 phase 3 trials who had no reported history of prior immunosuppressant use.
Methods: The sample for this post hoc analysis consisted of a subset of patients from TULIP-1 and TULIP-2 trials who were ‘immunosuppressant-naïve’, defined as patients with a treatment history at enrollment of antimalarial or oral GCs, or the combination of antimalarials and GCs, but without recorded history of prior use of immunosuppressants or DMARDs. DORIS remission attainment was assessed for the period after randomization. DORIS remission was defined as total clinical SLEDAI-2K score (sum of all SLEDAI-2K items except increased DNA binding and low complement) =0, physician global assessment < 0.5, and prednisone/equivalent dosage ≤5 mg/day. DORIS attainment was analyzed using a stratified Cochran–Mantel–Haenszel approach.
Results: A total of 257 patients (anifrolumab 300 mg, n=127; placebo, n=130) in the pooled TULIP-1 and TULIP-2 trial dataset were immunosuppressant naïve. Among this subgroup, 21 (16.2%) of immunosuppressant-naïve patients in the anifrolumab 300 mg group attained DORIS remission at Week 52 compared with 7 (6.0%) of immunosuppressant-naïve patients in the placebo group (treatment difference: 10.2% [95% CI: 1.3–19.1], nominal P=0.0242). DORIS attainment rates generally increased from baseline through to Week 52 (Figure 1).
Conclusion: In this post hoc analysis, a higher proportion of immunosuppressant-naïve patients with moderate to severe SLE treated with anifrolumab attained DORIS remission at Week 52 compared with those who received placebo. These results are consistent with EULAR recommendations to consider initiating treatment with biologics early, before immunosuppressants.
References
1. Fanouriakis A, et al. Ann Rheum Dis. 2019;78(6):736–45.
2. Furie RA, et al. Lancet Rheumatol. 2019;1(4):e208–e19.
3. Morand EF, et al. N Engl J Med. 2020;382(3):211–21.
To cite this abstract in AMA style:
Andrea d, Van Vollenhoven R, Morand E, Lindholm C, Hedberg J, Waratani M, Kielar D. DORIS Remission in Patients with SLE Treated with Anifrolumab: Post Hoc Analysis from TULIP-1 and TULIP-2 Trials in Patents with No Reported History of Prior Immunosuppressant Use [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/doris-remission-in-patients-with-sle-treated-with-anifrolumab-post-hoc-analysis-from-tulip-1-and-tulip-2-trials-in-patents-with-no-reported-history-of-prior-immunosuppressant-use/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/doris-remission-in-patients-with-sle-treated-with-anifrolumab-post-hoc-analysis-from-tulip-1-and-tulip-2-trials-in-patents-with-no-reported-history-of-prior-immunosuppressant-use/