Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Remission is considered the treatment goal in the management of patients with rheumatoid arthritis (RA). The objective of this analysis was to determine if low disease activity (LDA) at 6 months is a predictor of remission at 12 months in patients with RA treated with infliximab or golimumab in a Canadian real–world setting.
Methods: BioTRAC is an ongoing, prospective registry of patients initiating treatment for rheumatoid arthritis (RA), ankylosing spondylitis (AS), or psoriatic arthritis (PsA) with infliximab or golimumab as first biologics or after having been treated with a biologic for less than six months. Patients with RA treated with infliximab or golimumab who were enrolled between 2002 and 2012 and had 12 months of follow-up were included in this analysis.
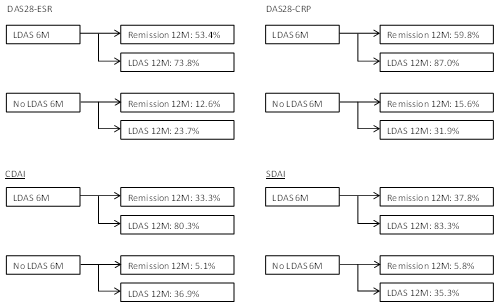
Results: A total of 436 patients with a mean (SD) age of 56.1 (13.1) years and disease duration of 10.4 (9.9) years were included in the analyses. Mean (SD) DAS-ESR, DAS-CRP, CDAI, and SDAI at baseline were 5.7 (1.5), 5.3 (1.3), 34.3 (16.0), and 36.9 (16.7), respectively. At 12 months 25.3%, 32.5%, 15.9% and 16.5% had DAS-ESR, DAS-CRP, CDAI and SDAI remission, respectively. Significant predictors of DAS-ESR remission at 12 months were LDA at 6 months [OR (95%CI) = 7.9 (4.5, 14.1)], change in SJC [OR (95%CI) = 1.1 (1.0, 1.1)] and DAS-ESR [OR (95%CI) = 0.8 (0.7, 1.0)] at 6 months. For DAS-CRP remission at 12 months, significant predictors were LDA at 6 months [OR (95%CI) = 8.0 (4.3, 14.9)] and change in DAS-CRP [OR (95%CI) = 0.7 (0.6, 0.9)] at 6 months. For CDAI remission at 12 months, significant predictor was LDA at 6 months [OR (95%CI) = 9.4 (4.6, 19.4)]. For SDAI remission at 12 months, significant predictor was LDA at 6 months [OR (95%CI) = 9.9 (4.3, 22.8)]. See Figure 1. Changes in CDAI, SDAI, TJC and SJC from baseline to 6 months were not associated with CDAI and SDAI remission at 12 months.
Conclusion: The results of this Canadian longitudinal observational study have shown that LDA at six months is a significant predictor of remission at 12 months. For patients achieving a low disease activity state at 6 months, there is a 7.9 to 9.9 odds ratio of achieving remission at 12 months. Data from this real-world registry suggest that a significant proportion of patients with LDA who had not achieved a therapeutic target of remission at 6 months do so at 12 months while maintained on the same biologic treatment.
Figure 1: Proportion of LDA Patients at 6 Months in LDA and Remission at 12 Months
Disclosure:
P. Baer,
Janssen Pharmaceutica Product, L.P.,
5;
W. G. Bensen,
None;
A. Chow,
JNJ, Amgen, Pfizer, Abbvie, UCB, Eli Lilly, Celgene, Takeda, Astra Zeneca, BMS, Roche,
5;
R. Y. Faraawi,
None;
D. Choquette,
None;
I. Fortin,
None;
J. T. Kelsall,
None;
D. E. Sholter,
None;
E. Rampakakis,
None;
J. S. Sampalis,
None;
F. Nantel,
None;
A. J. Lehman,
Janssen Canada,
3;
M. Shawi,
Janssen Canada,
3;
S. M. Otawa,
Janssen Canada,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/does-low-disease-activity-at-six-months-predict-remission-at-12-months-in-rheumatoid-arthritis-patients-treated-with-biologics-in-a-real-world-setting/