Session Information
Date: Monday, November 11, 2019
Title: Epidemiology & Public Health Poster II: Spondyloarthritis & Connective Tissue Disease
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Tumour necrosis factor inhibitors (TNFi) are efficacious in patients with psoriatic arthritis (PsA), but some patients switch to a different TNFi because of adverse events (AE) or lack of effect (LOE). The EuroSpA Collaboration has previously demonstrated a 1-year retention rate of 77% and 6 months LUNDEX adjusted 28-joint count Disease Activity Score (DAS28) remission rates of 45%1 in patients with PsA initiating the first TNFi treatment. Little is known about the effectiveness of switching to a second and third TNFi in patients with PsA.
We aim to investigate retention and remission rates at 6, 12 and 24 months in patients with PsA initiating the 2nd and 3rd TNFi in clinical practice across Europe. Secondly, to investigate whether the outcomes are associated with the reason for withdrawal (AE or LOE) from the previous TNFi-treatment.
Methods: Prospectively collected data on PsA patients in routine care from 12 European registries were pooled. Kaplan-Meier estimation was used to investigate TNFi retention rates. LUNDEX adjusted2 remission rates were calculated for DAS28< 2.6 and 28 joint Disease Activity index for Psoriatic Arthritis (DAPSA28) ≤4. Group comparisons were performed by Chi-square test.
Results: A total of 4971 patients initiating their 2nd TNFi and 1768 patients initiating their 3rd TNFi were included. Baseline characteristics are shown in the Table.
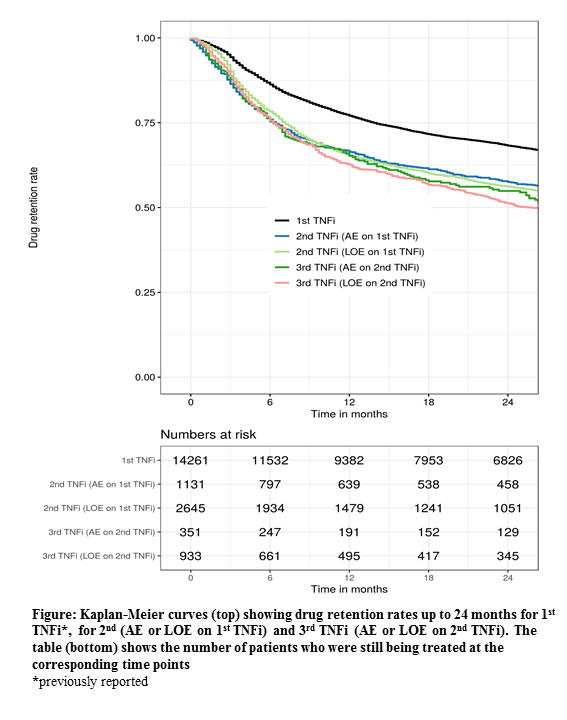
The overall retention rates for 2nd and 3rd TNFi at 12 months were 69% (67-70%) and 66% (64-68%), (2nd vs 3rd p=0.053), respectively (Figure). Corresponding retention rates for the individual registries ranged from 48-100% and 49-91%, respectively. If patients had stopped the 1st TNFi due to AE or LOE, 12-month retention rates for the 2nd TNFi treatment were 66% and 65%, respectively. In patients who stopped the 2nd TNFi due to AE or LOE, 12-month retention rates for the 3rd TNFi treatment were 65% and 63%, respectively.
For the 2nd and 3rd TNFi, 6 months LUNDEX adjusted DAS28 remission rates were 35% and 27% (p< 0.001), respectively, and for DAPSA28 remission 14% and 10% (p=0.008) (Table).
Conclusion: The EuroSpA Collaboration demonstrated decreasing retention and remission rates with increasing number of previous TNFi, although with only minor difference between 2nd and 3rd. Patients who had withdrawn from the previous TNFi due to LOE had retention rates and remission rates similar to those who had withdrawn due to AE.
Acknowledgement
Novartis Pharma AG and IQVIA for supporting the EuroSpA collaboration.
References
1Brahe et al. ACR 2018
2Arthritis Rheum, 2006, 54(2), p:600-6
To cite this abstract in AMA style:
Brahe C, Midtbøll Ørnbjerg L, Jacobsson L, Nissen M, Kristianslund E, Mann H, Santos M, Pombo-Suarez M, Nordström D, Rotar Z, Gudbjornsson B, Onen F, Codreanu C, Loft A, Lindström U, Moeller B, Sexton J, Pavelka K, Barcelos A, Sánchez-Piedra C, Eklund K, Tomsic M, Love T, Can G, IONESCU R, van de Sande M, van der Horst-Bruinsma I, Jones G, Iannone F, Michelsen B, Hyldstrup L, Krogh N, Østergaard M, Lund Hetland M. Does Drug Effectiveness of 2nd and 3rd TNF Inhibitors in Patients with Psoriatic Arthritis Depend on the Reason for Withdrawal from the Previous Treatment? – Results from the EuroSpA Research Collaboration [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/does-drug-effectiveness-of-2nd-and-3rd-tnf-inhibitors-in-patients-with-psoriatic-arthritis-depend-on-the-reason-for-withdrawal-from-the-previous-treatment-results-from-the-eurospa-research/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/does-drug-effectiveness-of-2nd-and-3rd-tnf-inhibitors-in-patients-with-psoriatic-arthritis-depend-on-the-reason-for-withdrawal-from-the-previous-treatment-results-from-the-eurospa-research/