Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Clinical trials leading to approval of the COVID19 vaccines did not include immunocompromised individuals. Concerns have been raised that immunogenicity of the vaccines may be impaired in patients with AIIRD on immunomodulatory drugs. The ACR COVID-19 Vaccine Clinical Guidance Task Force has suggested that the timing and dosing of certain drugs be modified to optimize vaccine response but acknowledged it was a “living document” that would likely change as additional studies were published. We sought to characterize antibody responses to the SARS COV-2 spike protein in patients with AIIRD both in those who adjusted and in those who failed to make adjustments to their drug therapy in a real world study.
Methods: We measured SARS CoV2 IgG antibody levels to the receptor-binding domain of the S1 spike antigen using a semi-quantitative assay (Siemens Atellica; QUEST) in patients with AIIRD followed in a single specialty community based practice of 13 providers (AOCC) in Charlotte NC from 3/17 – 5/14/2021. Patients with a prior self-reported history of COVID19 were excluded. All tests were performed by Day 14 or later following BNT162b2 (Pfizer), mRNA -1273 (Moderna) or Ad26COV2.S (Johnson & Johnson) vaccine. We compared the number of antibody positive (Ab+) vs. antibody negative(Ab-) and mean Ab levels in Ab+ patients for those who did or did not make adjustments to dosing/timing of methotrexate (MTX), JAK inhibitors (JAKi), rituximab (RTX) and/or abatacept (ABA). Associations were evaluated using Student’s t-test. In 12/2020, our clinic recommended adjustments to the dosing/timing of these drugs that were similar but slightly more stringent than subsequent recommendations in 2/2021 from the ACR Task Force (Table 1).
Results: 198 patients with AIIRD were studied. Baseline characteristics including gender, age, vaccine type and diagnosis are presented in Table 2. Findings related to % of total patients on MTX, JAKi, RTX and ABA with absent antibody response and comparison of antibody response with or without adjustment in dosing and/or timing are presented in Table 3. Only 13% of all patients on RTX and 52 % of all patients on ABA were Ab+. In contrast, fully 91% of patients on JAKi were Ab+ whereas 80% of patients on methotrexate alone were Ab+. Adjustments to the dose of MTX or JAKi did not significantly alter the immunogenicity as measured by IgG antibodies to the S1 spike antigen. Adjustments to the timing of vaccine in relationship to last RTX dose and last vaccine injection in relation to next RTX infusion failed to increase the likelihood of an antibody response. Adjustments to the timing of ABA (either iv or sc) did not significantly increase antibody response. Limitations to this real world study include a non-randomized design and not measuring potential residual confounders.
Conclusion: Holding JAKi, lowering the dose of MTX or altering the timing of the vaccine in relation to RTX or ABA administration did not alter immunogenicity as measured by SARS CoV2 Abs to the S1 spike antigen. Coupled with additional measure of humoral immune response and T cell function, these findings may provide further guidance for dosing and timing of immunomodulatory therapies in the setting of COVID19 vaccinations in patients with AIIRD.
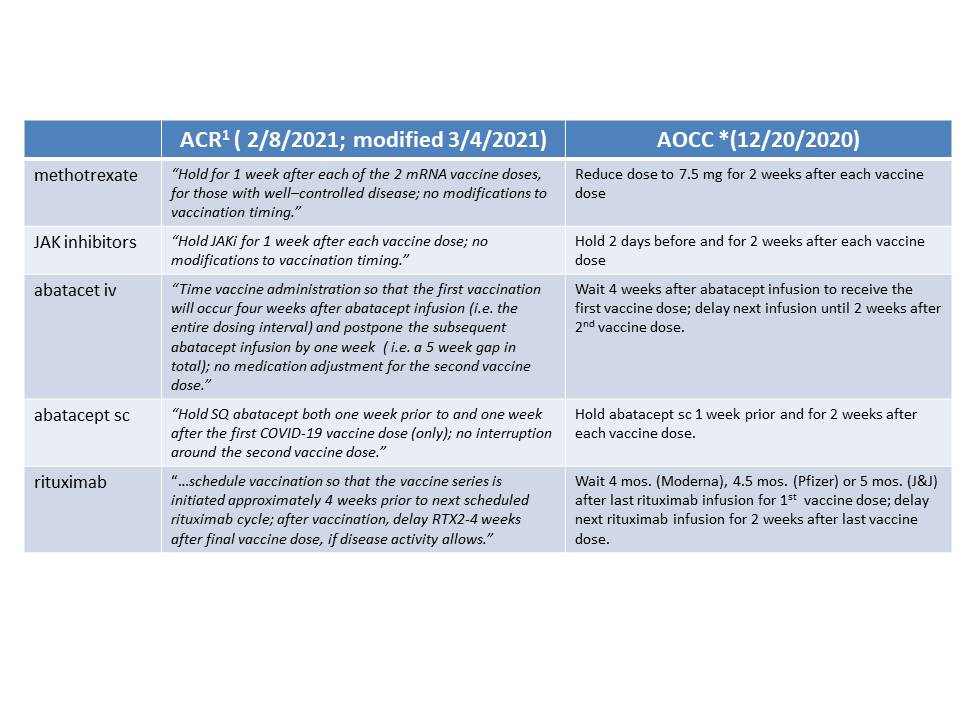 Table 1: Recommendations regarding adjustment of dosing/timing. *AOCC: Arthritis & Osteoporosis Consultants of the Carolinas, Charlotte, NC 1https://www.rheumatology.org/Portals/0/Files/COVID_19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf
Table 1: Recommendations regarding adjustment of dosing/timing. *AOCC: Arthritis & Osteoporosis Consultants of the Carolinas, Charlotte, NC 1https://www.rheumatology.org/Portals/0/Files/COVID_19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf
 Table 2: Baseline patient characteristics
Table 2: Baseline patient characteristics
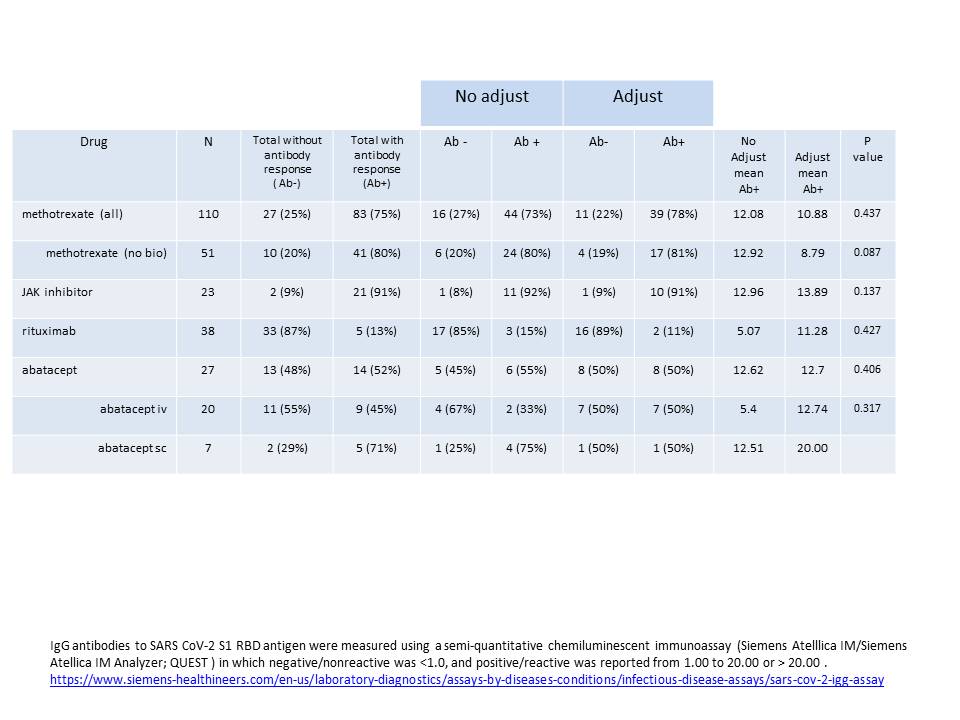 Table 3:Adjustments to drug dosing/timing. IgG antibodies to SARS CoV_2 S1 RBD antigen were measured using a semi-quantitative chemiluminescent immunoassay (Siemens Atelllica IM/Siemens Atellica IM Analyzer; QUEST ) in which negative/nonreactive was < 1.0, and positive/reactive was reported from 1.00 to 20.00 or > 20.00 . https://www.siemens-healthineers.com/en-us/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/sars-cov_2-igg-assay
Table 3:Adjustments to drug dosing/timing. IgG antibodies to SARS CoV_2 S1 RBD antigen were measured using a semi-quantitative chemiluminescent immunoassay (Siemens Atelllica IM/Siemens Atellica IM Analyzer; QUEST ) in which negative/nonreactive was < 1.0, and positive/reactive was reported from 1.00 to 20.00 or > 20.00 . https://www.siemens-healthineers.com/en-us/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/sars-cov_2-igg-assay
To cite this abstract in AMA style:
Laster A, Lam G, McCarter S, Gladue H, Kashif A, Siceloff E, Lackey V, Robertson C, Toci A, Calabrese L. Does Adjustment to Dosing and Timing of Immunomodulatory Drugs Impact Immunogenicity of COVID19 Vaccines in Patients with Autoimmune and Inflammatory Rheumatic Disease (AIIRD)? [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/does-adjustment-to-dosing-and-timing-of-immunomodulatory-drugs-impact-immunogenicity-of-covid19-vaccines-in-patients-with-autoimmune-and-inflammatory-rheumatic-disease-aiird/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/does-adjustment-to-dosing-and-timing-of-immunomodulatory-drugs-impact-immunogenicity-of-covid19-vaccines-in-patients-with-autoimmune-and-inflammatory-rheumatic-disease-aiird/
