Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Marketing of cheaper biosimilar biological agents has created financial incentives for switching from the corresponding originator drugs (=non-medical switch). The economic benefit might potentially be outweighed by extra costs due to patient education or closer monitoring. However, only few studies have previously explored the use of health care resources associated with non-medical switching.1 In year 2016, Danish national guidelines recommended switch of patients with inflammatory rheumatic diseases treated with originator etanercept (ETA) to biosimilar SB4 in routine care.2 We aimed to explore if switching lead to increased health care utilization and costs.
Methods: Observational cohort study. Adult patients who switched from ETA to SB4 were identified in the Danish nationwide DANBIO registry. In the National Patient Registry we identified health utilization (hospital admissions/hospital days/outpatient visits/prescription medication use) and comorbidities. Estimation of health utilization included average use and costs one year before/after the switch, changes after the switch, and whether patient characteristics affected changes. Analyses were by adjusted 2-step gamma distributed regression models, and for changes over time a GEE model was applied. Impact of comorbidities was explored as interaction terms in the model. Medication costs of ETA and SB4 were not included in model, because these drugs are provided by the hospital department responsible for the therapy and not by private pharmacies.
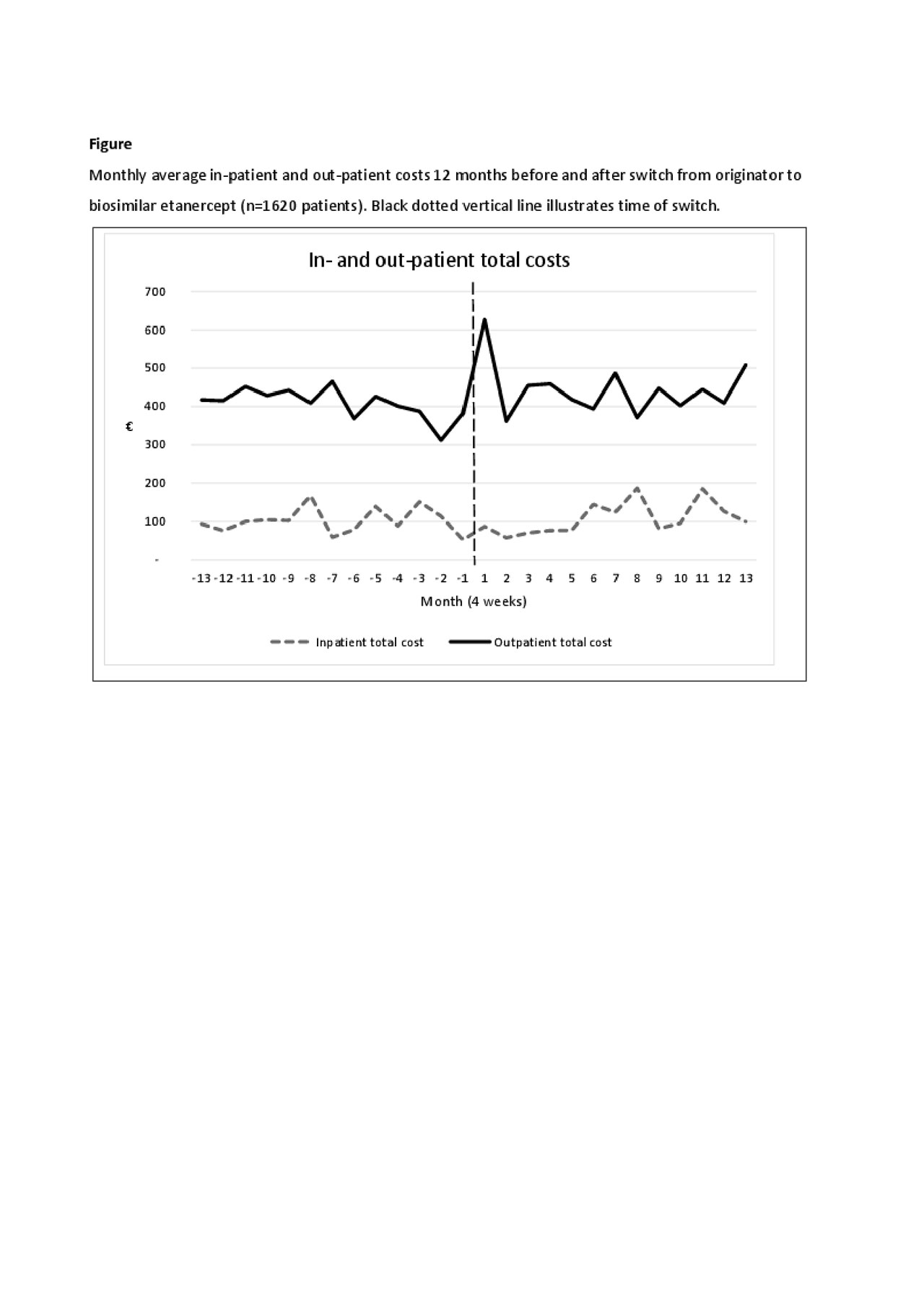
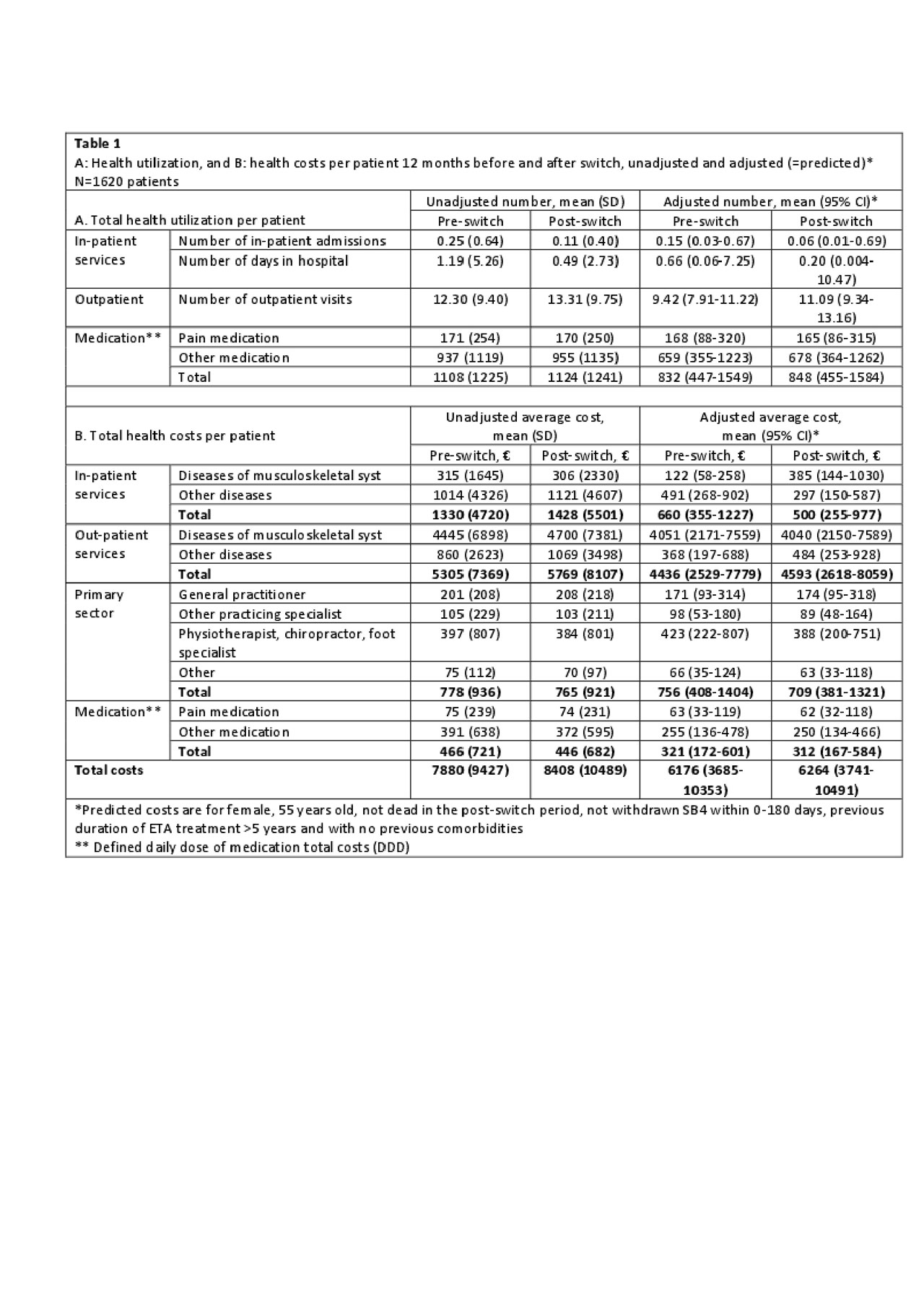
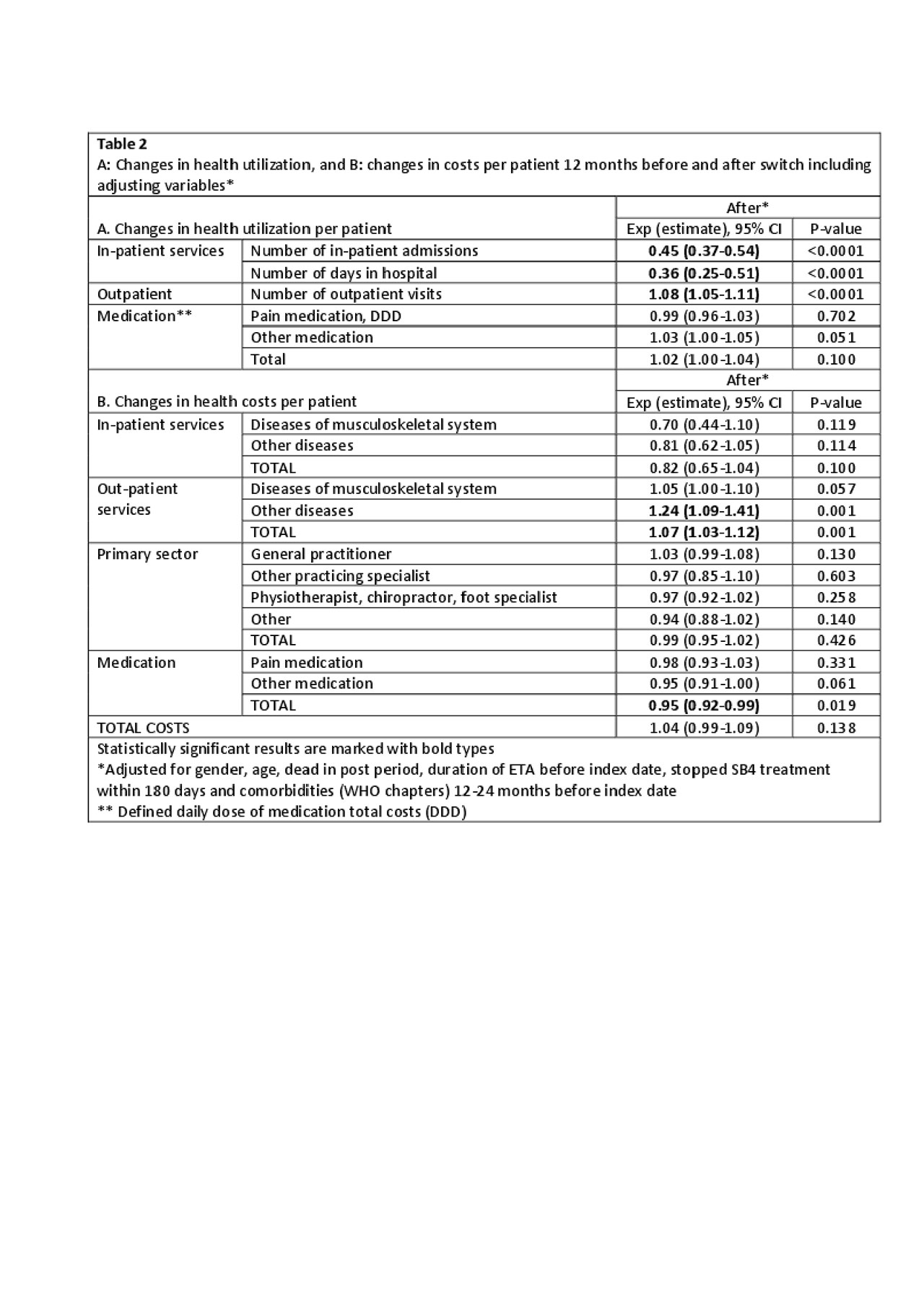
Results: 1620 patients were included (mean age 55 years(SD 14.7), 40% male). Costs before and after switching were mainly driven by outpatient visits (67% and 72% of all costs, respectively) (Table 1). In general, the adjusted health costs were lower than the unadjusted costs e.g. number of in-patient admissions, hospital days and in-patient costs due to the adjustment for previous comorbidities in the regression (Table 1). Monthly fluctuations of costs were similar before/after switch (Figure). After switching, an increase was observed in the use (8%) and costs (7%) of outpatient services. Number of in-patient days and number of days in hospital decreased (Table 2), but the total number of patients with in-patient services was low (Table 1). Medication costs decreased (5%) (Table 2). Patients with longer ETA treatment duration had increased number of outpatient visits after the switch, higher out-patient total costs and costs related to general practitioners. Higher age (3% per year increase) was associated with higher in-patient total costs related to diseases of the musculoskeletal system and connective tissue diseases. Gender and comorbidities had no impact on costs before and after the switch.
Conclusion: We demonstrated no obvious changes in overall use and costs of health care services following a mandatory non-medical switch from originator to biosimilar etanercept. Longer ETA treatment duration and higher age were associated with increased use and costs of some services.
Reference List
(1) Glintborg B, et al. RMD Open 2018; 4(2):e000710.
(2) Glintborg B, et al. ARD. 2019 Feb;78(2):192-200
To cite this abstract in AMA style:
Glintborg B, Ibsen R, Qwist Bilbo R, Lund Hetland M, Kjellberg J. Does a Mandatory Non-medical Switch from Originator to Biosimilar Etanercept Lead to Increase in Healthcare Use and Costs? A Danish Register-based Study of 1620 Patients with Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/does-a-mandatory-non-medical-switch-from-originator-to-biosimilar-etanercept-lead-to-increase-in-healthcare-use-and-costs-a-danish-register-based-study-of-1620-patients-with-inflammatory-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/does-a-mandatory-non-medical-switch-from-originator-to-biosimilar-etanercept-lead-to-increase-in-healthcare-use-and-costs-a-danish-register-based-study-of-1620-patients-with-inflammatory-arthritis/