Session Information
Date: Tuesday, October 23, 2018
Title: 5T106 ACR Abstract: Measures of Healthcare Quality I: QI in RA (2856–2861)
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Routine measurement of RA disease activity and adjustment of drug therapy to attain remission or low disease activity is recommended by the ACR and EULAR. However, it is unclear whether this occurs in real-world settings. We identified longitudinal RA treatment changes stratified by disease activity categories and the methods used to measure it.
Methods: Using the ACR’s national EHR-based RISE registry, we identified adult RA patients who had ≥ 1 rheumatologist visit (index visit) with a disease activity measure available (e.g. RAPID3, CDAI) in 2016. We assessed disease activity measurement(s) used for each patient and calculated the proportion of patients with moderate/high disease activity (M/HDA) at the index visit. We evaluated treatment and disease activity changes at the follow-up visit occurring month 7-12 after index. Results were stratified based on available measurement tool and patients’ baseline RA medications. Subgroup analyses were conducted for patients with ‘persistent’ M/HDA (at both the index visit and the visit immediately prior), for patients with past (ever) biologic use, for patients with seropositive RA, and for those with past methotrexate use.
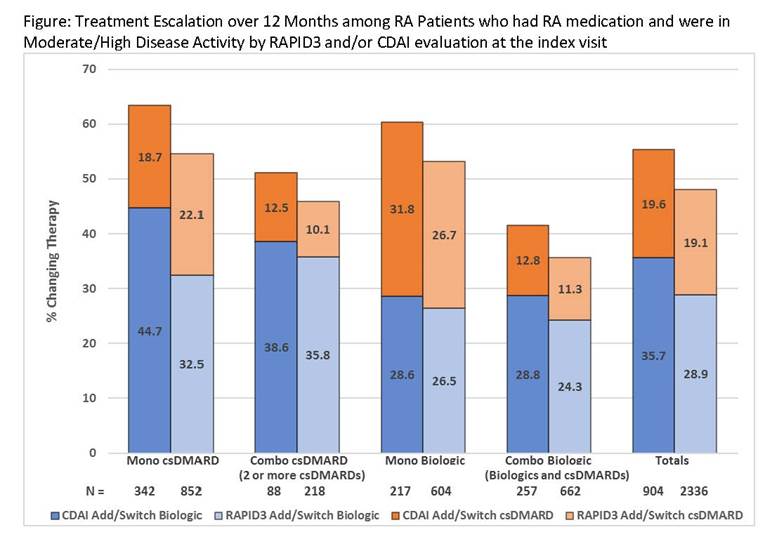
Results: Among 457,950 patients included in the 2016 RISE registry, we identified 50,996 eligible RA patients. Mean age was 62.4 (SD: 13.7); 76.7% were women; 52.8% had Medicare insurance and 25.3% had concurrent glucocorticoid use. Most (85%) were evaluated with only one RA measurement at the index visit. RAPID3 was most commonly used (79%), followed by CDAI (34%) and DAS 28 ESR/CRP (3%). A total of 7,467 (14.6%) patients had both RAPID3 and CDAI measured at the index visit. For patients with M/HDA and RA medication at the index visit and who had a follow-up visit occurring 7-12 months after index (n=2,336 for RAPID3, n=904 for CDAI), changes in treatment is shown (Figure). Irrespective of baseline RA medication, RA treatment change did not exceed 65% of the patients. For the subgroup of patients with persistent M/HDA by RAPID3 (n=1,241) or CDAI (n=497), the proportion of treatment switching was consistent with the main analysis. Patients with any history of biologic exposure had a similar pattern in therapy change (<10% different). The treatment pattern for seropositive patients and with a history of methotrexate was also similar to the main analysis.
Conclusion: Irrespective of the measurement tool used (RAPID3 or CDAI), past biologic use, or persistent M/HDA, almost half of RA patients in the ACR RISE registry with M/HDA did not change their current therapy over the next year. To optimize RA therapies in accordance with treat-to-target principles, more effective intervention is needed to encourage treatment change and improve patient outcomes.
To cite this abstract in AMA style:
Yun H, Chen L, Xie F, Patel H, Boytsov N, Zhang X, Curtis JR. Do Patients with Moderate or High Disease Activity Escalate RA Therapy According to Treat-to-Target Principles? Results from the Acr’s RISE Registry [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/do-patients-with-moderate-or-high-disease-activity-escalate-ra-therapy-according-to-treat-to-target-principles-results-from-the-acrs-rise-registry/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/do-patients-with-moderate-or-high-disease-activity-escalate-ra-therapy-according-to-treat-to-target-principles-results-from-the-acrs-rise-registry/