Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Childhood-onset systemic lupus erythematosus (cSLE) is associated with symptoms such as fatigue, pain, and depressive symptoms that contribute to poor health-related quality of life. The Treatment and Education Approach for Childhood-Onset Lupus (TEACH; a cognitive behavioral therapy (CBT) program) has been shown in our prior work to significantly reduce fatigue and depressive symptoms when compared with standard care. The current study is a secondary data analysis of this recently completed trial which explores disease-related outcomes following TEACH, including disease activity, medication adherence, and health-related quality of life.
Methods: We conducted a randomized controlled trial of 59 youth ages 12-22 years meeting the ACR diagnostic classification criteria for SLE, from six rheumatology sites across the U.S. and Canada. Eligible participants had elevations in at least one target symptom area (e.g., fatigue, pain, or depression). Participants completed a baseline assessment, were randomized to 1) TEACH, a remotely delivered six-week CBT program with medical treatment as usual (TAU), or 2) TAU alone, then completed a post-assessment 8 weeks later. The current study explored: i) physician-reported disease activity via the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and a Visual Analog Scale (VAS, range 0-100); ii) medication adherence measured by the Medication Adherence Self-Report Inventory (MASRI, range 0-100%); and iii) health-related quality of life measured by Pediatrics Quality of Life – Adolescent version (PedsQL-A, 0-100). Independent samples t-tests compared changes from baseline to post-assessment between those who received TEACH+TAU vs. those who received TAU alone.
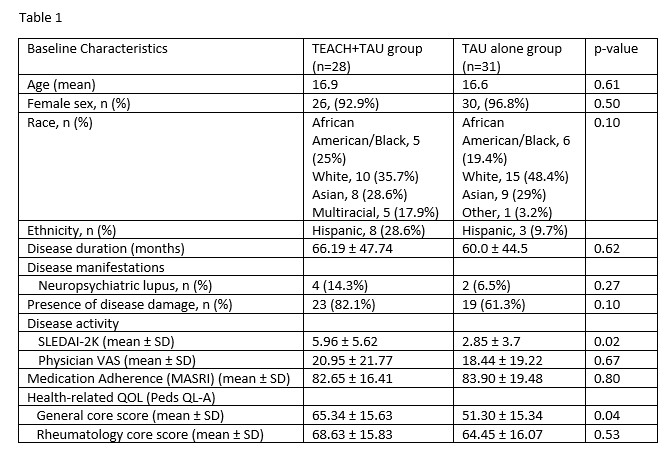
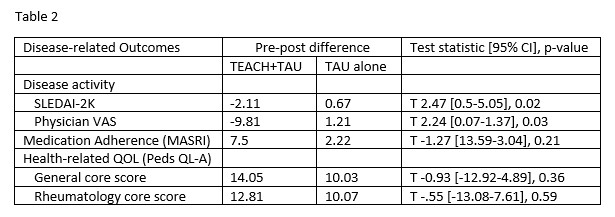
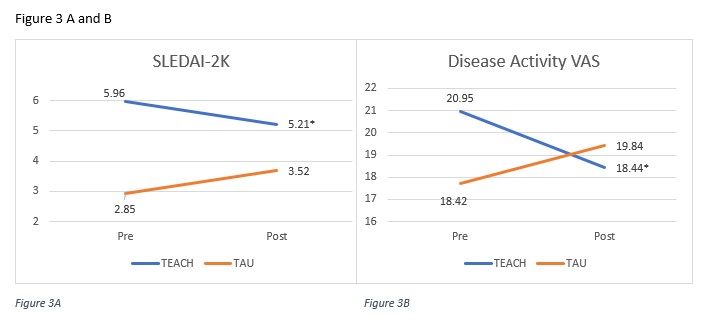
Results: Twenty-eight (47.5%) participants received TEACH+TAU, and 31 (52.5%) received TAU alone. Participant characteristics are shown in Table 1. At baseline, disease activity (SLEDAI-2K) and health-related quality of life (general core score) were higher in the TEACH+TAU group. The change in disease activity (SLEDAI-2K) scores between groups significantly differed, with the TEACH+TAU group demonstrating a significant decrease in disease activity scores (t(40) = 2.47, p = 0.02; Table 2, Figure 3A). There was also a statistically significant decrease in physician-reported disease activity VAS for the TEACH+TAU group vs the TAU group, (t(38) = 2.24, p = 0.031; Figure 3B). There were no other statistically significant differences between groups for changes in medication adherence or health-related quality of life.
Conclusion: TEACH may be associated with short-term improvement in disease activity, in addition to reducing depressive symptoms and fatigue. TEACH represents a promising treatment modality to mitigate mental health symptoms and improve disease outcomes. Future research will examine the long-term effectiveness of TEACH when implemented into real-world pediatric rheumatology clinics.
To cite this abstract in AMA style:
Cunningham N, Adler M, Danguecan A, Reid M, Ely S, Reeves M, Ng L, Moaf P, El Tal T, Mossad S, Flores Pereira L, Levy D, Hiraki L, Stinson J, Ahola Kohut S, abulaban k, Kessler E, Allen S, Rubinstein T, Rothschild E, Rosenwasser N, Nanda K, Canny S, Smitherman E, Huie L, Birmingham J, Ogbu E, Brunner H, Sharma D, Thompson A, Thompson J, Moyer M, Nguyen E, Chapson A, Knight A. Disease-Related Outcomes of Cognitive Behavioral Therapy in Randomized Control Trial for Youth with Childhood-onset SLE: A Secondary Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/disease-related-outcomes-of-cognitive-behavioral-therapy-in-randomized-control-trial-for-youth-with-childhood-onset-sle-a-secondary-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-related-outcomes-of-cognitive-behavioral-therapy-in-randomized-control-trial-for-youth-with-childhood-onset-sle-a-secondary-analysis/