Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: High placebo responses pose challenges to systemic lupus erythematosus (SLE) clinical trials.1This analysis aims to identify predictors of standard of care plus placebo (SOC+PBO) response using data from epratuzumab (Emab; a humanized anti-CD22 monoclonal antibody) and dapirolizumab pegol (DZP; a polyethylene glycol-conjugated antigen-binding fragment lacking a functional Fc domain that inhibits CD40L) trials.2,3
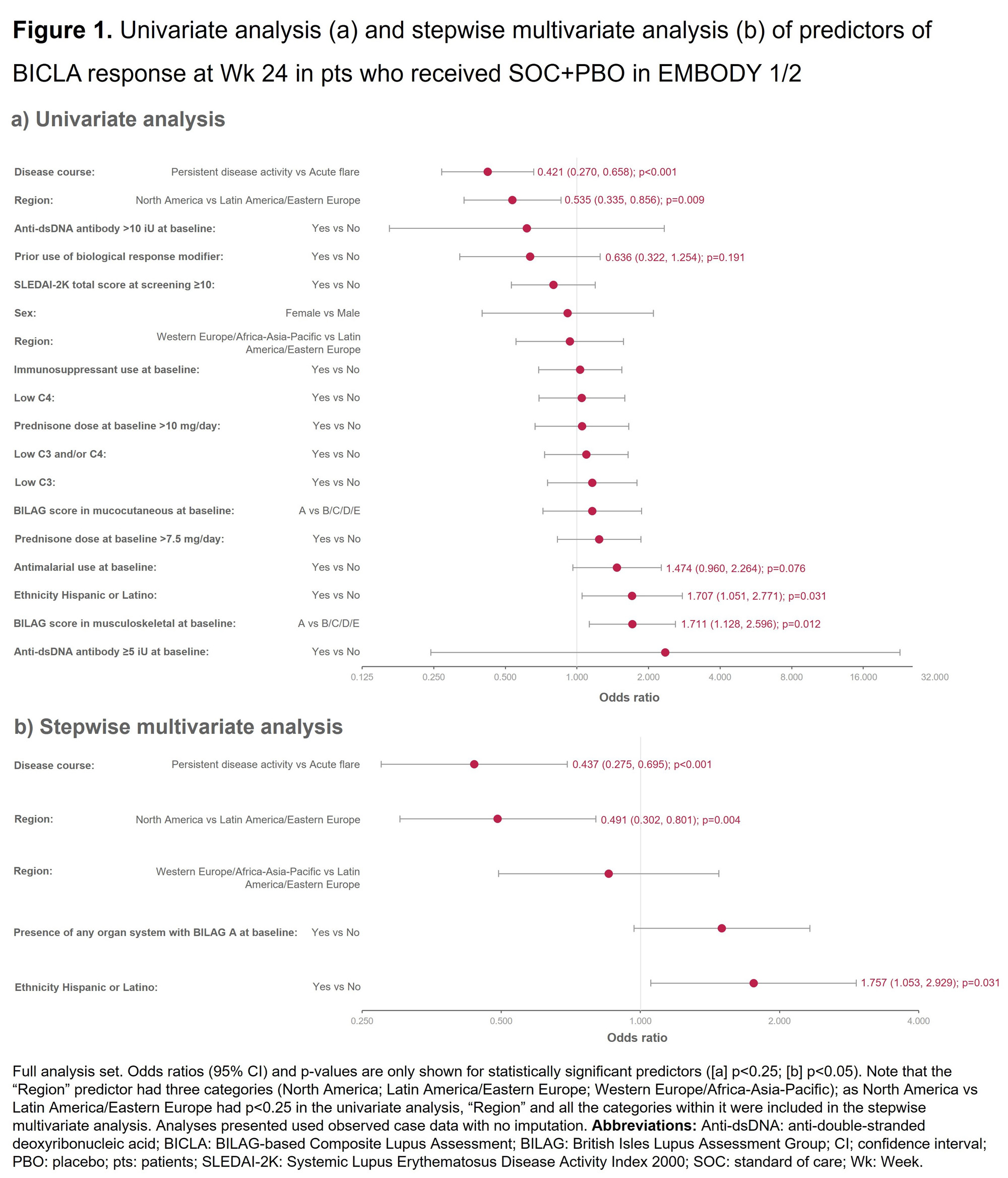
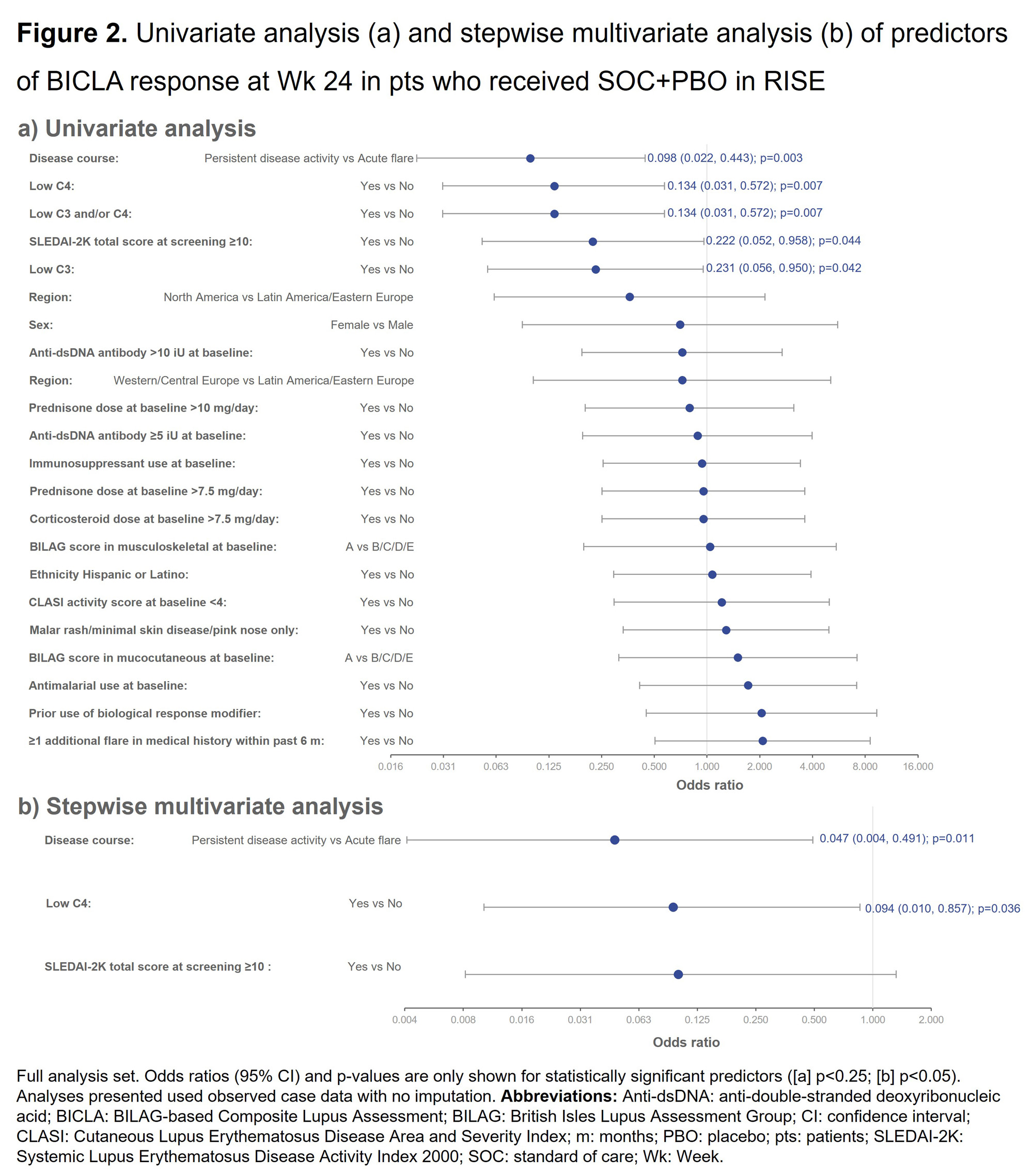
Methods: Analyses were performed on the full analysis sets of the SOC+PBO arms of two phase 3 trials of Emab (EMBODY 1 [NCT01262365]; n=249, and EMBODY 2 [NCT01261793]; n=263) and the phase 2b trial of DZP (RISE [NCT02804763]; n=43) in SLE.2,3 Response was assessed by British Isles Lupus Assessment Group (BILAG)-based Composite Lupus Assessment (BICLA) response at Week (Wk) 24.4 Univariate/multivariate analyses were performed, with 18 and 22 potential predictors of SOC+PBO response evaluated in EMBODY 1/2 and RISE, respectively. Stepwise multivariate analysis included univariate predictors with p< 0.25, using p≤0.1 as entry/stay criteria. In subgroup analyses, disease course at screening was defined using BILAG 2004 item level scores as acute flare (worsening/new symptoms) or persistent disease (symptoms rated as the same) based on the past 4 wks compared with the 4 wks prior to those; low C3/C4 was defined as below the lower limit of normal at screening.
Results: Univariate and subsequent stepwise multivariate analysis found the following significant (p < 0.05) predictors of BICLA response at Wk 24 in EMBODY 1/2: acute flare (vs persistent disease activity; p< 0.001), Latin America/Eastern European (vs North America; p=0.004), and Hispanic or Latino (vs not; p=0.031; Figure 1). Significant (p < 0.05) predictors of BICLA response at Wk 24 in RISE following stepwise multivariate analysis were: acute flare (vs persistent disease activity; p=0.011) and normal C4 (vs low C4; p=0.036; Figure 2).
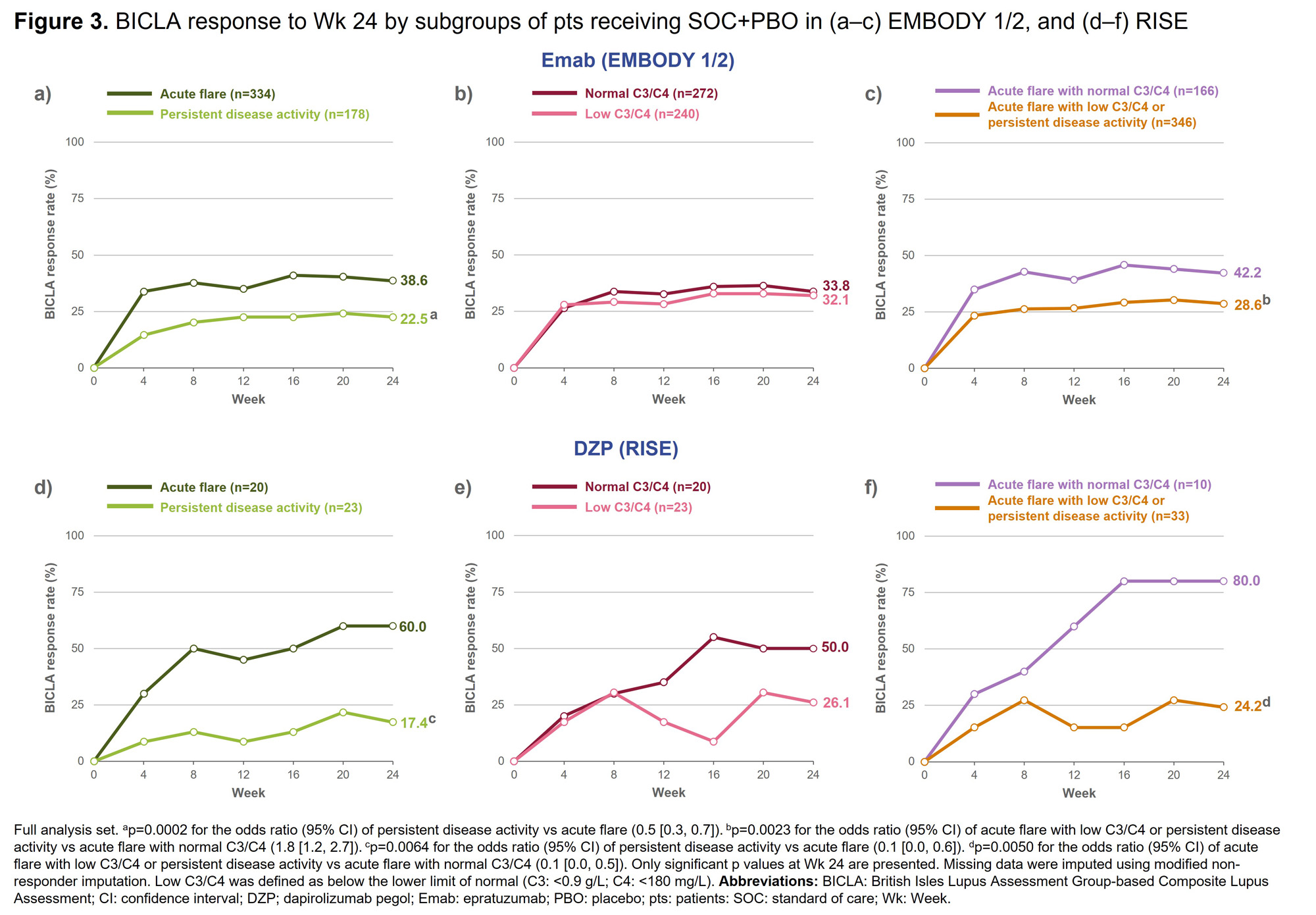
Focusing on disease characteristics, recent acute flares and normal complement were identified as predictors of SOC+PBO response. In all trials, a significantly higher proportion of patients (pts) with acute flare (vs persistent disease activity) achieved BICLA response (Figure 3a& 3d). In RISE, a significantly higher proportion of pts with normal C3/C4 (vs low C3/C4) achieved BICLA response (Figure 3e); this pattern was not observed in EMBODY 1/2 (Figure 3b). In all trials, when the subgroups were combined, a significantly higher proportion of pts with acute flare and normal C3/C4 (vs acute flare with low C3/C4 or persistent disease activity) achieved BICLA response (Figure 3c & 3f).
Conclusion: In both EMBODY 1/2 and RISE, acute flare predicted SOC+PBO response. In RISE, normal complement levels also predicted SOC+PBO response; low complement has been described as a predictor of increased treatment effect in previous studies.5 Recent medical history of pts may have to be considered when defining SLE study populations.
References: 1. Durcan L. Lancet. 2023;S0140-6736(23)00342-2.2. Clowse ME. Arthritis Rheumatol. 2017;69(2):362–75. 3. Furie RA. Rheumatology (Oxford). 2021;60:5397–407. 4. Wallace D. Arthritis Rheum. 2011;63(Suppl 10):S885. 5. van Vollenhoven RF. Ann Rheum Dis. 2012;71(8):1343–9.
To cite this abstract in AMA style:
Stach C, Gordon C, Taieb V, Stojan G, Merrill J. Disease Course and Complement as Predictors of Response to Standard of Care Plus Placebo in Patients with SLE: A Post Hoc Analysis of Dapirolizumab Pegol and Epratuzumab Clinical Trial Data [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/disease-course-and-complement-as-predictors-of-response-to-standard-of-care-plus-placebo-in-patients-with-sle-a-post-hoc-analysis-of-dapirolizumab-pegol-and-epratuzumab-clinical-trial-data/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-course-and-complement-as-predictors-of-response-to-standard-of-care-plus-placebo-in-patients-with-sle-a-post-hoc-analysis-of-dapirolizumab-pegol-and-epratuzumab-clinical-trial-data/