Session Information
Date: Sunday, November 12, 2023
Title: (0252–0282) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Treatment of autoinflammatory periodic diseases (AID) with the interleukin-1β inhibitor canakinumab (CAN) has been shown to be safe and effective in controlled trials and real-world setting. In the RELIANCE registry, long-term safety and efficacy of CAN in patients with cryopyrin-associated periodic syndromes (CAPS), familial Mediterranean fever (FMF), hyper-IgD syndrome/mevalonate kinase deficiency (HIDS/MKD) and tumor necrosis factor receptor-associated periodic syndrome (TRAPS) on CAN therapy were investigated in routine clinical practice.
Methods: The RELIANCE registry is a prospective, non-interventional, observational study in Germany enrolling pediatric (age ≥2 years) and adult patients with a clinically confirmed diagnosis of AID who routinely receive CAN. Efficacy and safety parameters are recorded at baseline and assessed at 6-month intervals. To compare disease control between indications, parameters of 30 months visits were analyzed.
Results: In the present interim analysis, data were included from a total of n=232 patients with a diagnosis of CAPS, FMF, TRAPS and HIDS/MKD enrolled in the RELIANCE registry between October 2017 and December 2022. The median age of the total study cohort was 20.0 years (2−80 years). Most patients (n=198, 85 %) were CAN pre-treated when entering the study and the median duration of total CAN treatment before and during the study was 4 years (0−15 years).
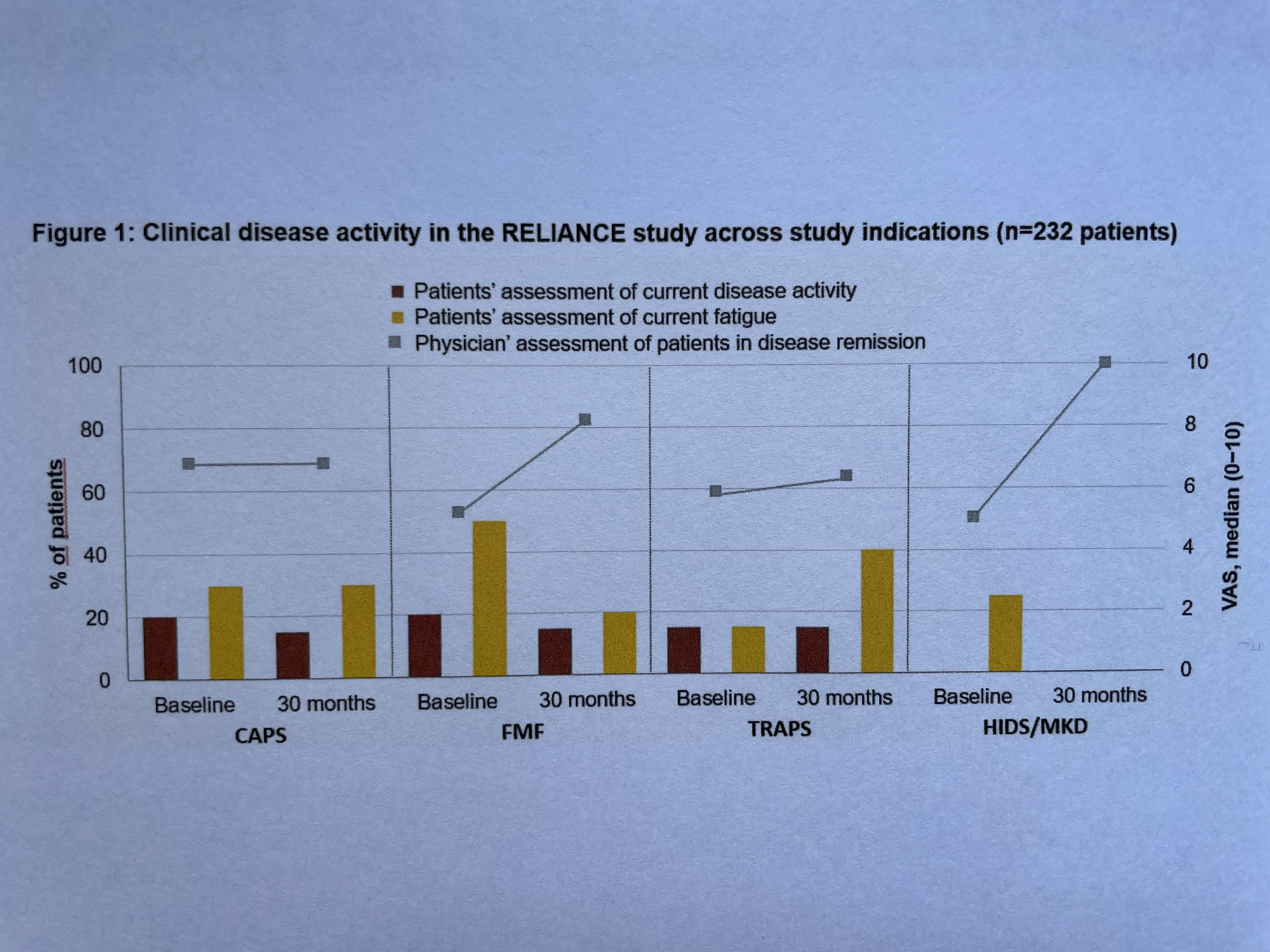
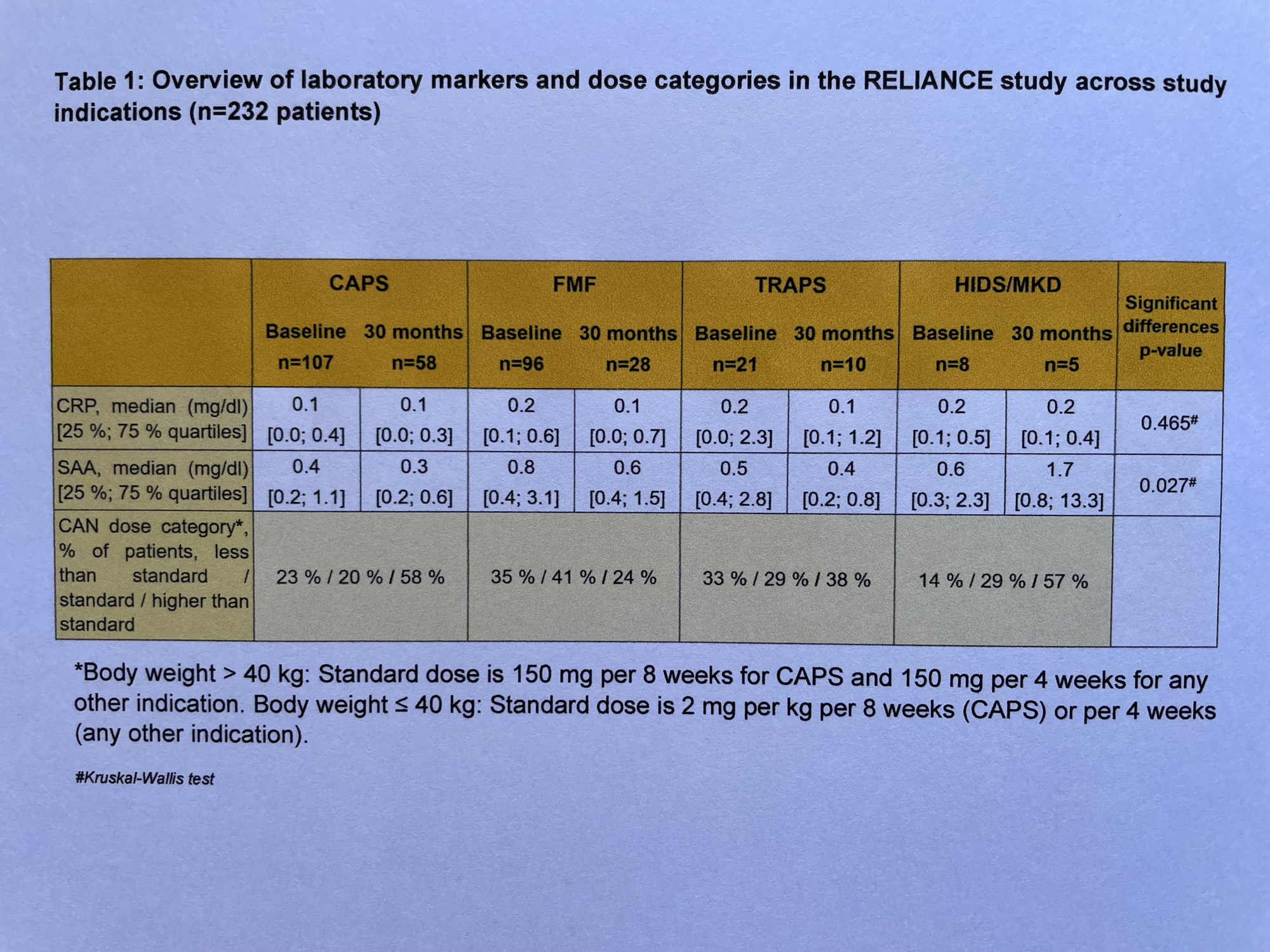
Of 58/28/10/5 CAPS/FMF/TRAPS/HIDS patients with month 30 visits documented, 68/82/63/100% were in disease remission according to physician assessment. Patient-reported median disease activity and fatigue were low (1.5/1.5/1.5/0 and 3/2/4/0 on a 0−10 VAS scale). Inflammation markers (median) were within the limits of normal including neutrophil counts (2975/3420/3262/2897 n/µL). Statistical analysis confirmed similar efficacy across diseases in most parameters.
Even though these outcomes suggest an adequate disease control, an impact of the disease on patient´s social life was reported in 38/22/50/50% and negative influence on mood in 26/6/0/50% of patients.
Conclusion: The interim data of the RELIANCE study confirm sustained disease control of long-term treatment with CAN across all study indications. Even though disease activity measures suggest adequate disease control, patients´ social life and mood were negatively impacted by the disease.
To cite this abstract in AMA style:
Foeldvari I, Kallinich T, Blank N, Henes J, Kortus-Goetze B, Oommen P, Pankow A, Krickau T, Schuetz C, Horneff G, Rech J, Weller-Heinemann F, Janda A, Hufnagel M, Meier F, Dressler F, Borte M, Andreica I, Wasiliew P, Fiene M, Windschall D, Krusche M, Kuempfel T, Weber-Arden J, Kuemmerle-Deschner J. Disease Control in Patients with Monogenetic Autoinflammatory Diseases Under Canakinumab Treatment – Comparison of 30 Months Interim Data from the RELIANCE Registry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/disease-control-in-patients-with-monogenetic-autoinflammatory-diseases-under-canakinumab-treatment-comparison-of-30-months-interim-data-from-the-reliance-registry/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-control-in-patients-with-monogenetic-autoinflammatory-diseases-under-canakinumab-treatment-comparison-of-30-months-interim-data-from-the-reliance-registry/