Session Information
Date: Tuesday, November 10, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment Poster III: Therapy
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Apremilast (APR), an oral
phosphodiesterase 4 inhibitor, acts to regulate immune responses in psoriatic
arthritis (PsA). PALACE 3 compared the efficacy and safety of APR with placebo (PBO)
in patients with active PsA, including active skin disease, despite prior conventional DMARDs and/or biologics. We
report the efficacy and safety of APR treatment over 104 weeks in PALACE 3.
Methods: Patients were randomized (1:1:1) to PBO,
APR 30 mg BID (APR30), or APR 20 mg BID (APR20) stratified by baseline DMARD
use (yes/no) and psoriasis involvement of the body surface area (<3%/≥3%).
The PBO-controlled phase
continued to Week 24, with an early escape option at Week 16. At Week 24, all
remaining PBO patients were re-randomized to APR30 or APR20. Double-blind APR treatment continued
to Week 52; subsequently, patients could continue APR for up to 4 additional
years in an open-label extension study.
Results: 505 patients were randomized and received
≥1 dose of study medication (PBO: n=169;
APR30: n=167; APR20: n=169);
82% of patients entering
the second year of APR therapy completed 104 weeks of treatment. Patients receiving APR at Week 104 demonstrated
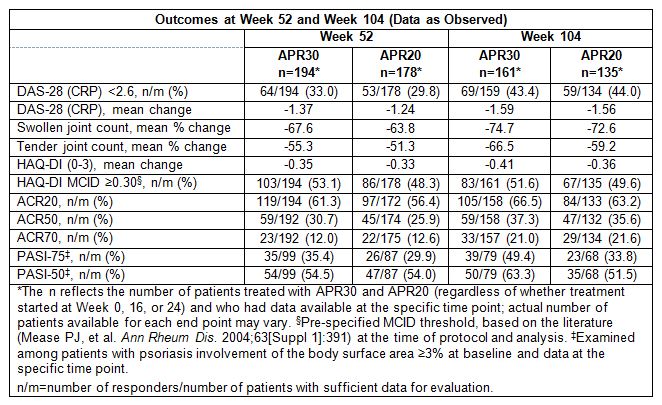
sustained decreases in disease activity. At baseline, 65.7% and 27.7% of APR30 patients
and 61.8% and 28.6% of APR20 patients had moderate (DAS-28 [CRP]): ≥3.2
to ≤5.1) and severe (DAS-28 [CRP]: >5.1) disease, respectively; 43.4% (APR30)
and 44.0% (APR20) achieved DAS-28 (CRP) remission at Week 104 (Table). Sustained
relief of signs/symptoms and improvements in physical function were
demonstrated by the swollen/tender joint count mean percent change, HAQ-DI mean
change, proportion of patients with HAQ-DI score exceeding the minimal
clinically important difference (MCID) ≥0.30 threshold, modified
ACR20/ACR50/ACR70 response rates, and PASI-75/PASI-50 response rates (Table). No
new safety concerns were observed with treatment through Week 104; long-term
findings indicate that APR tolerability improved with long-term exposure. During Weeks >52 to ≤104, adverse
events (AEs) occurring in
≥5% of APR-exposed patients were nasopharyngitis and upper respiratory
tract infection; most AEs were mild/moderate in severity. Serious AEs occurred
in 5.8% (APR30) and 6.2% (APR20) during Weeks 0 to ≤52 and 8.7% (APR30) and 7.5% (APR20) over Weeks >52 to
≤104. Fewer discontinuations due to AEs occurred during Weeks >52 to
≤104 (3.5%) than during Weeks 0 to ≤52 (7.5%).
Conclusion: Over 104 weeks, APR demonstrated sustained,
clinically meaningful improvements in PsA disease activity, physical function,
and associated psoriasis. APR continued to demonstrate a favorable safety
profile and was generally well tolerated.
To cite this abstract in AMA style:
Edwards CJ, Blanco FJ, Crowley J, Hu C, Shah K, Birbara CA. Disease Activity and Safety during Long-Term (104-Week) Treatment with Apremilast, an Oral Phosphodiesterase 4 Inhibitor, in Patients with Psoriatic Arthritis: Results from a Phase III, Randomized, Controlled Trial and Open-Label Extension [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/disease-activity-and-safety-during-long-term-104-week-treatment-with-apremilast-an-oral-phosphodiesterase-4-inhibitor-in-patients-with-psoriatic-arthritis-results-from-a-phase-iii-randomized-co/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-activity-and-safety-during-long-term-104-week-treatment-with-apremilast-an-oral-phosphodiesterase-4-inhibitor-in-patients-with-psoriatic-arthritis-results-from-a-phase-iii-randomized-co/