Session Information
Date: Tuesday, October 23, 2018
Title: 5T106 ACR Abstract: Measures of Healthcare Quality I: QI in RA (2856–2861)
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Although national quality measures promote the measurement of disease activity (DA) in RA, the burden of DA in a population-based sample of RA patients is unknown. We used the ACR’s RISE registry to assess variation in DA and methods of DA measurement nationwide.
Methods: RISE is a national, EHR-enabled registry that passively collects data on all patients seen by participating practices, reducing the selection bias present in single-insurer claims databases. As of June 2017, RISE held validated data from 663 providers in 110 practices, representing ~19% of the U.S. clinical rheumatology workforce. Patients in this study had 2 RA codes ≥ 30 days apart and ≥ 1 RA DA score recorded from July 2016 – June 2017 by a practice with ≥ 20 RA patients. DA scores from validated instruments (CDAI, DAS, PASII, or RAPID3) were categorized using accepted cut-points. We calculated 1) the proportion of patients in remission, low, moderate, or high DA on their most recent assessment, overall and aggregated by practice or state; and 2) proportion with ≥2 DA scores ≥ 90 days apart with all assessments in remission/low DA. We then tested whether DA was associated with the measurement tool used using a chi square test and generated adjusted state-level proportions of patients in remission/low DA on the most recent assessment using logistic regression.
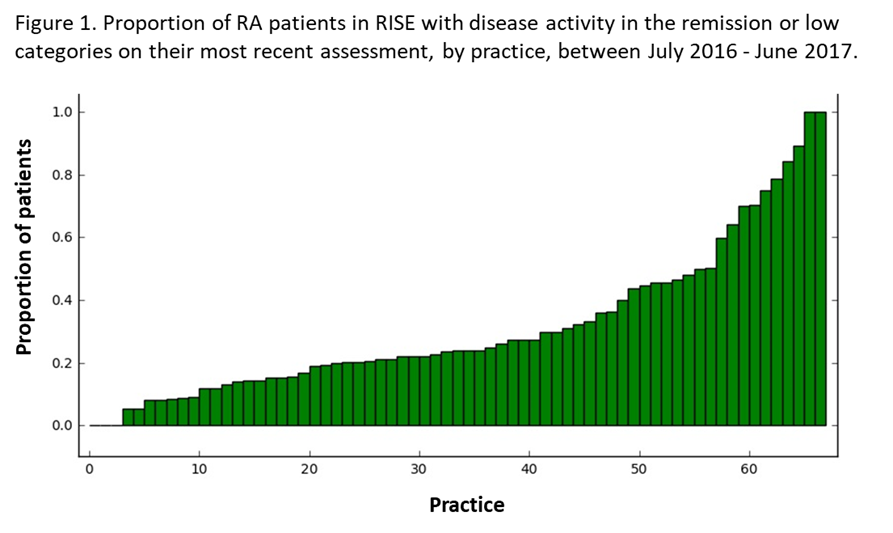
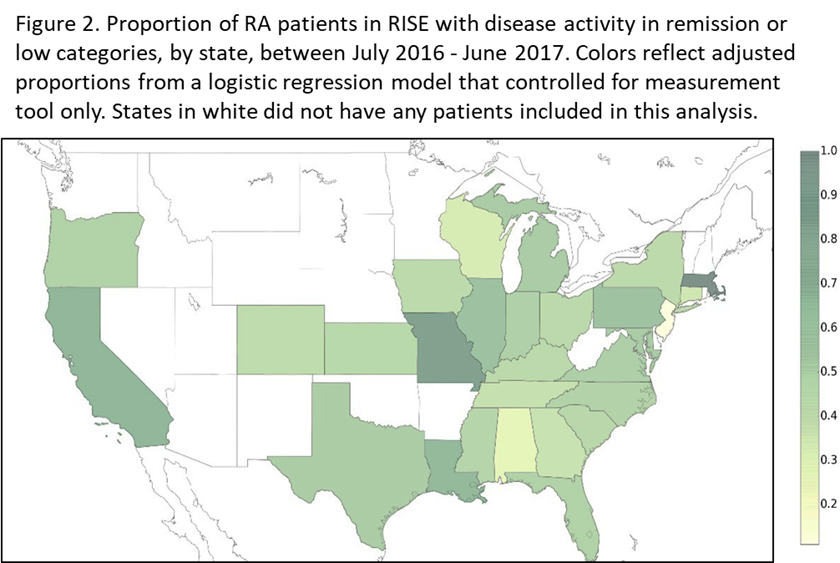
Results: We included 56,850 patients from 71 practices; 77% were female, 65% white, with mean age 61±13. Overall, 21% of patients were in remission and 24%, 29%, and 26% had low, moderate, and high DA, respectively. The proportion of patients in a given practice in remission/low DA ranged from 0-99% (Figure 1). Of the 35,573 patients with ≥ 2 DA scores, 27% were consistently in remission/low DA. RAPID3 was the most used tool (54%), followed by CDAI (42%), DAS (2%), and PASII (2%); RAPID3 scores were more likely to be moderate/high DA compared to CDAI scores (p<0.001). State-level variation was significant, even after adjusting for measurement tool (p<0.001, Figure 2).
Conclusion: Less than half of RA patients across the U.S. have DA in remission or low categories on their most recent assessment, with large differences across practices and states. Although some of this variation can be explained by the tool used to assess DA, additional factors such as underlying patient characteristics or preferences, availability and affordability of medications, or differences in treatments or treatment intensity should also be explored.
To cite this abstract in AMA style:
Schmajuk G, Evans M, Kay J, Suter LG, Clowse MEB, Morgan E, Reimold A, Limmani A, Johansson T, Lewis L, Yazdany J. Disease Activity and Its Measurement in Patients with RA across the U.S.: Data from the Rheumatology Informatics System for Effectiveness (RISE) Registry [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/disease-activity-and-its-measurement-in-patients-with-ra-across-the-u-s-data-from-the-rheumatology-informatics-system-for-effectiveness-rise-registry/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-activity-and-its-measurement-in-patients-with-ra-across-the-u-s-data-from-the-rheumatology-informatics-system-for-effectiveness-rise-registry/