Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic inflammatory disease of the joints and skin that affects 1/1000 people in the US. Tumor necrosis factor-alpha (TNF-α) inhibitors are used when symptoms are severe. However, current research describing TNF-α inhibitors persistence rates, defined as time from initiation to discontinuation of the drug, is inconsistent and incomplete. This study examined characteristics associated with persistence of and response to TNF-α inhibitors, as well as reasons for discontinuation.
Methods: US veterans enrolled in the Program to Understand the Longterm Outcomes in Spondyloarthritis (PULSAR) database across 11 medical centers in the United States from 2007 – 2017 who 1) were diagnosed with PsA or psoriasis by a rheumatologist or dermatologist and 2) had been treated with a TNF-α inhibitor were included in the study. Length of treatment with a biologic was determined using VA prescription data. Drug discontinuation was defined as the length between the usual dose of the TNF-α inhibitor plus 90 days without treatment. Course was defined as the difference between the date the prescription was first filled and date of discontinuation without >90-day gap in treatment. Reasons for agent discontinuation were documented into PULSAR by a provider at rheumatology appointments. The following reasons for discontinuation were included: primary failure (no efficacy within 6 months of treatment), secondary failure (loss of efficacy after 6 months of treatment), adverse event, concern for an adverse event, financial/access barrier, non-adherence, and other. Stata was used to conduct time-to-event and multivariate analyses.
Results: 321 individuals with 931 TNF-α inhibitor courses, including adalimumab (N = 381), certolizumab (N = 24), etanercept (N = 397), golimumab (N = 42), and infliximab (N = 87), were included in the study. The mean age was 55.4 years, and 252 of the 321 patients had a diagnosis of both PsA and psoriasis. 83.8% of the cohort continued at least one TNF-α inhibitor course at one year, and 64.8% continued at two years. On average, the probability of discontinuing a TNF-α inhibitor increased by 9.6% for each additional biologic trial (HR 1.096, p< 0.001). Former smoker (HR 0.827, p=0.037) and PsA duration in years (HR 0.991, p< 0.001) were also significantly correlated with discontinuation of a TNF-α inhibitor. On univariate and multivariate analyses, infliximab had a lower discontinuation rate than adalimumab (HR 2.114, p< 0.001), etanercept (HR 2.360, p< 0.001), and certolizumab (HR 3.048, p=0.001); however, the discontinuation rate of golimumab was lower than that of infliximab on univariate analysis only (HR 3.035, p< 0.001). The most commonly cited reason for discontinuing TNF-α inhibitor treatment was secondary failure (33%).
Conclusion: The majority of patients in this study continued at least one TNF-α inhibitor course at both 12 and 24 months. Secondary failure was the most prevalent reason for discontinuation, suggesting that further research should be conducted to examine reasons for loss of efficacy and immunogenicity of TNF-α inhibitors.
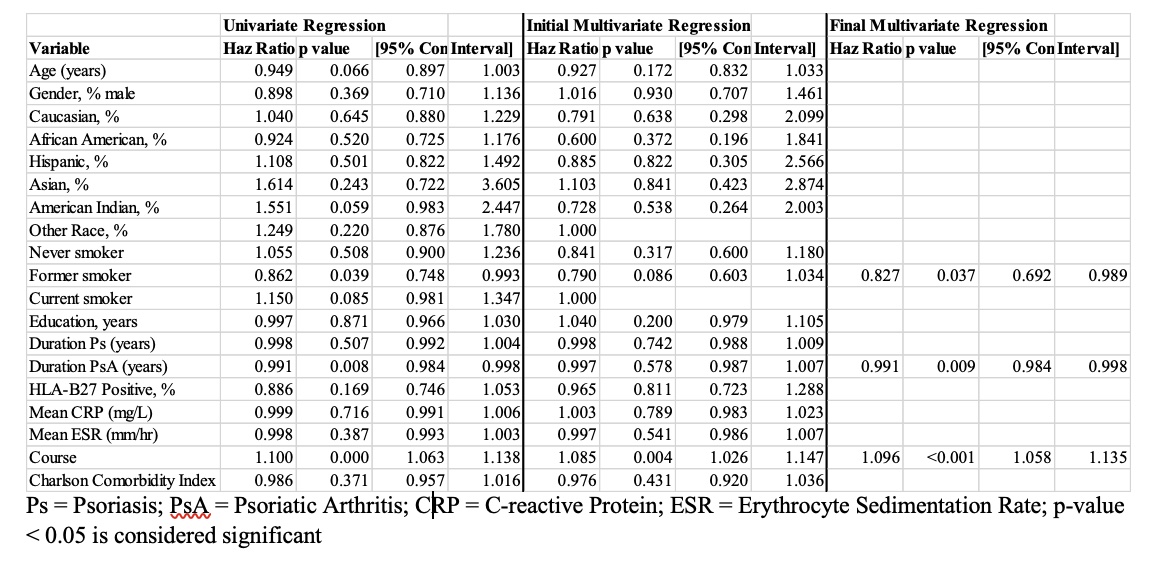 Table 1: Characteristics of TNF-α Inhibitor Discontinuation
Table 1: Characteristics of TNF-α Inhibitor Discontinuation
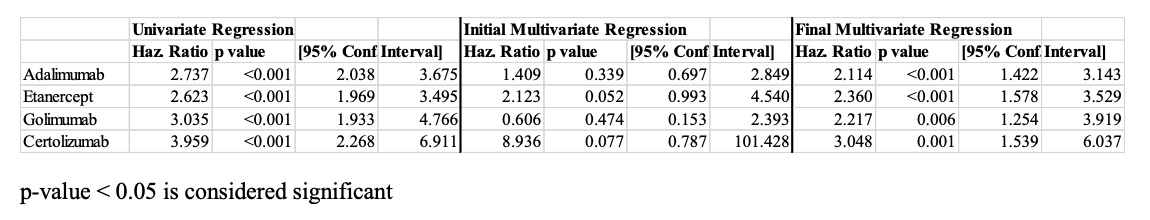 Table 2: Discontinuation of TNF-α Inhibitor Compared to Infliximab
Table 2: Discontinuation of TNF-α Inhibitor Compared to Infliximab
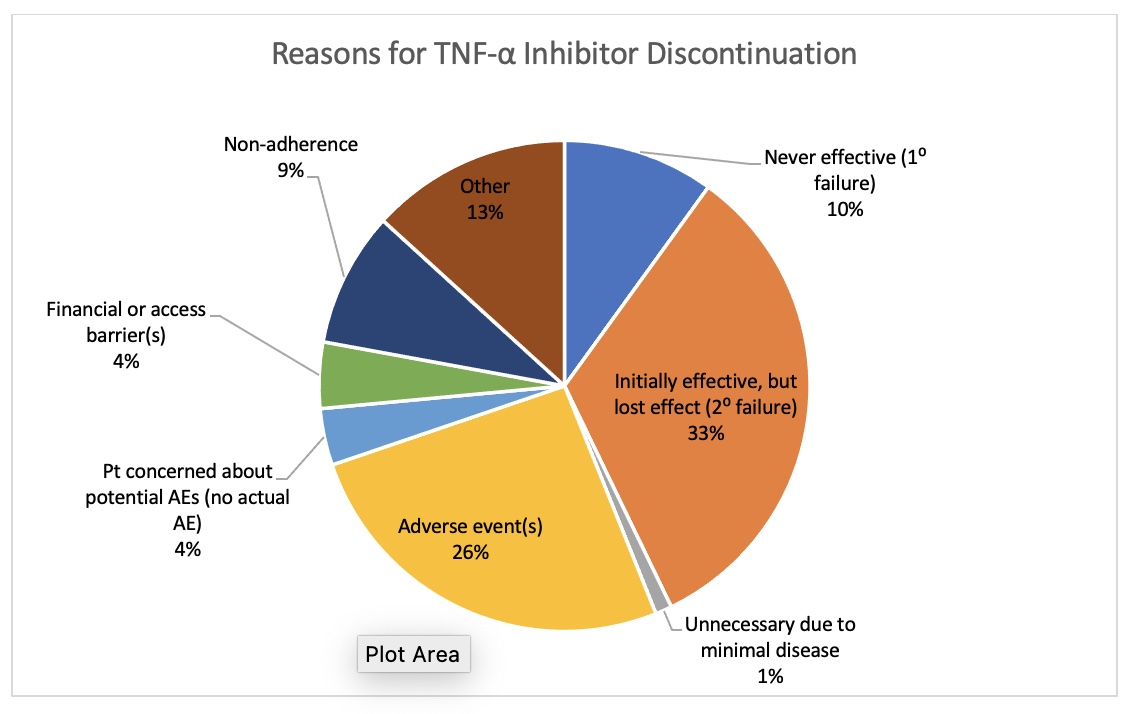 Figure 1: Reasons for TNF-α Inhibitor Discontinuation
Figure 1: Reasons for TNF-α Inhibitor Discontinuation
To cite this abstract in AMA style:
Wolfe S, Cheng E, Ganuthula K, Fang M, Kerr G, Walsh J, Chang E, Raychaudhuri S, Brees K, Dellavalle R, Reimold A, Caplan L. Discontinuation of Tumor Necrosis Factor Inhibitors in Psoriatic Arthritis and Psoriasis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/discontinuation-of-tumor-necrosis-factor-inhibitors-in-psoriatic-arthritis-and-psoriasis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-of-tumor-necrosis-factor-inhibitors-in-psoriatic-arthritis-and-psoriasis/
