Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Methotrexate and TNF inhibitors (TNFi) are commonly used in the treatment of RA, PsA, and other SpA. While MTX is a mainstay of RA treatment, its efficacy and tolerability have not been as well studied in spondyloarthritis. Our aims were 1) to assess the rates of MTX and TNFi discontinuation in patients with PsA and ankylosing spondylitis (AS) compared to patients with RA, hypothesizing that MTX discontinuation, but not TNFi discontinuation, would occur sooner in patients with PsA or AS; and 2) to determine whether concomitant methotrexate use was associated with later TNFi discontinuation in PsA and AS.
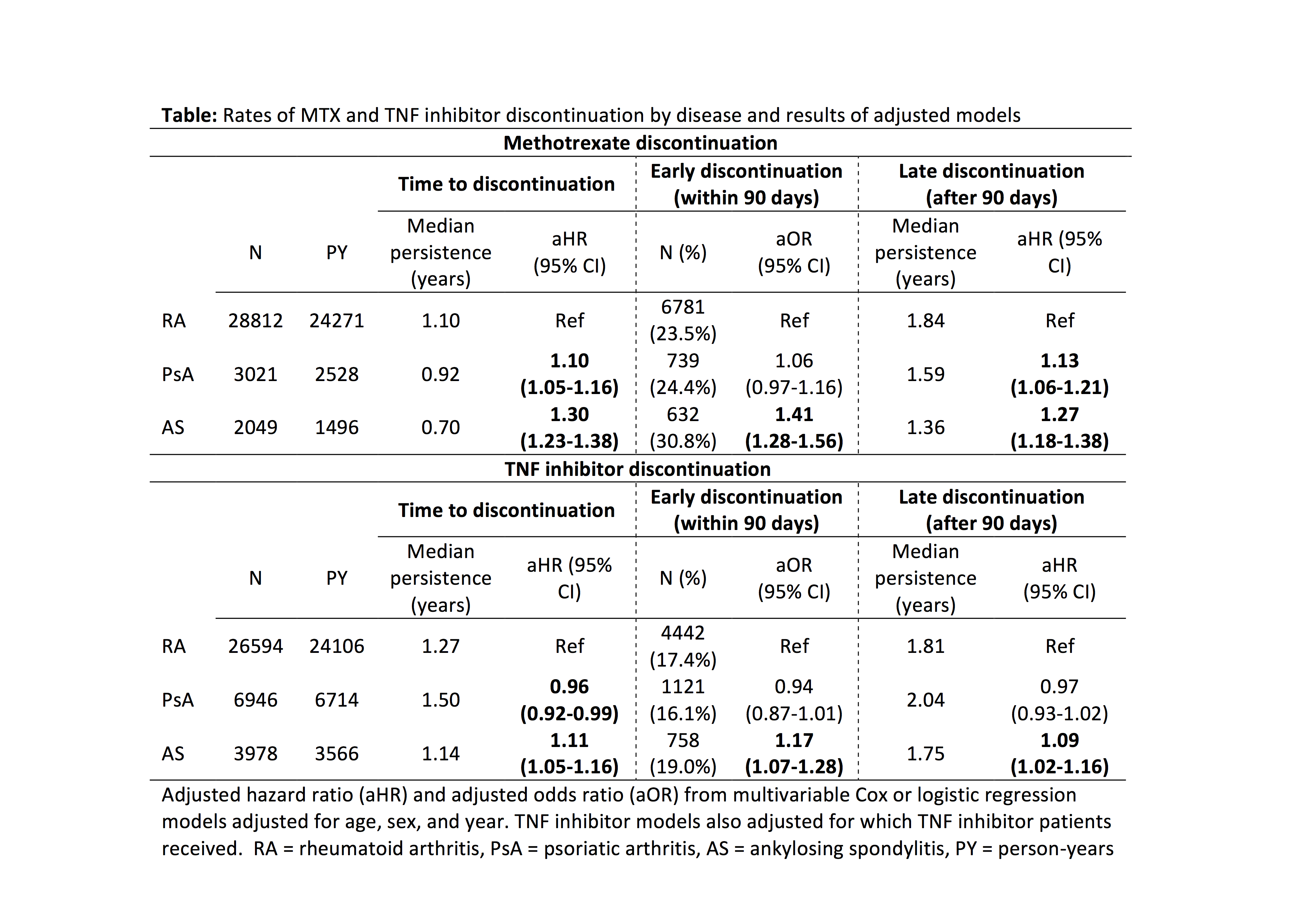
Methods: This retrospective study using OptumInsight administrative data 2000-2014 evaluated adults with RA, PsA, or AS (based on two diagnosis codes and DMARD use) with no prior biologic use who received a first ever MTX prescription or first ever TNFi prescription or infusion, requiring 6 months of preceding data (baseline) and at least 90 days of follow up. Cox proportional hazards were used to compare time to medication discontinuation over the next two years between patients with RA, PsA, or AS, adjusting for age, sex, and calendar year. We also assessed rates of early discontinuation (within 3 months) and late discontinuation (after 3 months).
Results: We identified 33,882 patients initiating MTX and 36,518 initiating a TNFi. Median MTX persistence was 1.05 years and 24% of patients discontinued MTX within 90 days. Discontinuation occurred sooner in patients with PsA [aHR 1.10 (1.05-1.16)] and AS [aHR 1.30 (1.23-1.38)] vs. RA (Table). Early MTX discontinuation was more common in AS, and late discontinuation was more common in both PsA and AS (Table). Median TNFi persistence was 1.29 years and 17% of patients discontinued within 90 days. TNFi discontinuation occurred sooner in patients with AS [aHR 1.11 (1.05-1.16)] but later in patients with PsA [aHR 0.96 (0.92-0.99)] vs. RA (Table). Associations between AS and sooner TNFi discontinuation, however, were attenuated and no longer significant after adjustment for concomitant medications, comorbidities, and healthcare utilization (not shown). Among TNFi initiators, concomitant use of MTX was associated with longer TNFi persistence in RA, PsA, and AS (all p < 0.001, p for interaction 0.85). Depression, anxiety, chronic pain, opioid use, and greater comorbidity burden were associated with sooner discontinuation of MTX and TNFi in all groups.
Conclusion: TNFi and especially MTX discontinuation are common, with greater discontinuation in patients with mental health disorders, comorbidities, and chronic pain. Patients with PsA and AS discontinue MTX sooner than patients with RA but continue TNFi at similar rates, possibly indicating poorer tolerability or efficacy of MTX in spondyloarthritis. Use of MTX, however, is associated with less TNFi discontinuation in all disease groups.
To cite this abstract in AMA style:
George MD, Baker J, Ogdie A. Discontinuation of Methotrexate or TNF Inhibitors in Patients with Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/discontinuation-of-methotrexate-or-tnf-inhibitors-in-patients-with-rheumatoid-arthritis-psoriatic-arthritis-and-ankylosing-spondylitis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-of-methotrexate-or-tnf-inhibitors-in-patients-with-rheumatoid-arthritis-psoriatic-arthritis-and-ankylosing-spondylitis/