Session Information
Date: Monday, November 14, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. In a post-hoc analysis of the tofacitinib RA long-term extension (LTE) studies, we evaluated the effect of discontinuation of MTX or glucocorticoids (GC) on the maintenance of clinical efficacy of tofacitinib.
Methods: Data were analyzed from 2 open-label studies (NCT00413699 [ongoing; March 2015 data-cut] and NCT00661661) of patients (pts) with RA who completed randomized Phase 1/2/3 tofacitinib studies and 3 years of the LTE studies (LTE baseline to Year 3). Pts in this analysis received tofacitinib 5 or 10 mg twice daily (BID) and utilized MTX or GC at LTE baseline. Discontinuation of MTX or GC was defined as no MTX/GC use within the last 30 days prior to the Year 3 visit. Clinical efficacy was evaluated at Year 3 utilizing the Clinical Disease Activity Index (CDAI); further assessment stratified patients by clinical response 3 months after entrance into the LTE studies and was performed for maintenance of response out to Year 3.
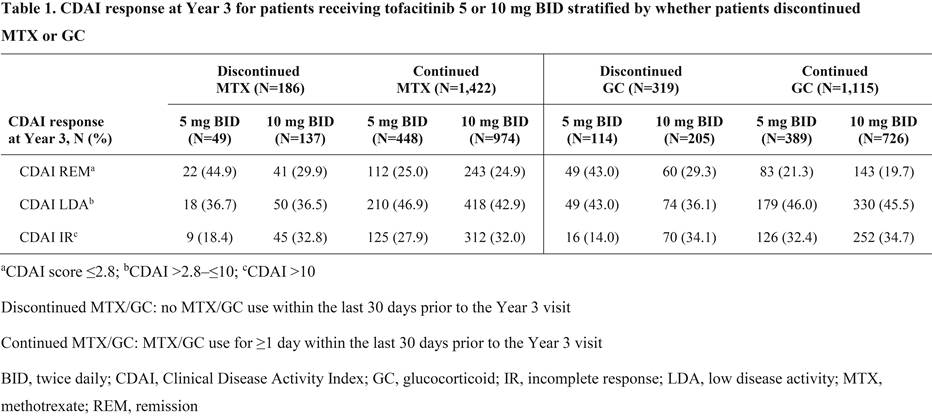
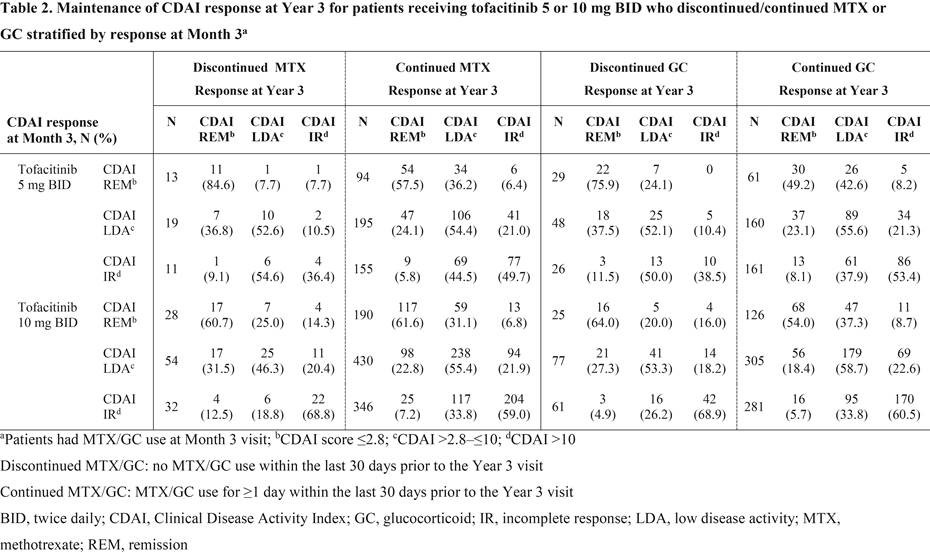
Results: Overall, 186/1,608 pts that received MTX at LTE baseline (11.6%; tofacitinib 5 mg BID: n=49; tofacitinib 10 mg BID: n=137) discontinued MTX and 319/1,434 pts that received GC at LTE baseline (22.2%; tofacitinib 5 mg BID: n=114; tofacitinib 10 mg BID: n=205) discontinued GC out to Year 3. Baseline demographics and disease characteristics for pts receiving tofacitinib 5 and 10 mg BID were generally similar irrespective of whether pts discontinued/continued MTX or GC. Most pts who discontinued MTX or discontinued GC achieved CDAI remission (≤2.8) or low disease activity (>2.8–≤10) vs CDAI incomplete response (>10) at Year 3 (Table 1). Mean (standard deviation) time off MTX and GC prior to Year 3, respectively, was 532 (347) and 523 (324) days for tofacitinib 5 mg BID and 593 (338) and 592 (337) days for tofacitinib 10 mg BID. Most pts maintained their 3-month response at Year 3 after the discontinuation of MTX or GC, and response rates were comparable with pts who did not discontinue MTX or GC after Month 3 (Table 2). Furthermore, of those not receiving MTX (1,044 pts) or GC (1,218 pts) at LTE baseline, 65 pts (6.2%; tofacitinib 5 mg BID: n=22; tofacitinib 10 mg BID: n=43) initiated MTX, and 306 pts (25.1%; tofacitinib 5 mg BID: n=82; tofacitinib 10 mg BID: n=224) initiated GC prior to Year 3.
Conclusion: Pts who achieve a high level response with tofacitinib may be able to discontinue MTX or GC and maintain their response. Additional analysis is warranted to understand the reasoning behind why pts initiated MTX or GC in the LTE studies.
To cite this abstract in AMA style:
Fleischmann R, Wollenhaupt J, Cohen S, Weinblatt M, Wang L, Fan H, Andrews J, Takiya L, Bananis E. Discontinuation of Methotrexate or Glucocorticoids in Patients with Rheumatoid Arthritis Treated with Tofacitinib: Clinical Efficacy Data from Long-Term Extension Studies [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/discontinuation-of-methotrexate-or-glucocorticoids-in-patients-with-rheumatoid-arthritis-treated-with-tofacitinib-clinical-efficacy-data-from-long-term-extension-studies/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-of-methotrexate-or-glucocorticoids-in-patients-with-rheumatoid-arthritis-treated-with-tofacitinib-clinical-efficacy-data-from-long-term-extension-studies/