Session Information
Date: Tuesday, November 15, 2016
Title: Spondylarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment - Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Clinical trials have demonstrated the efficacy of biologic therapy in improving the clinical and patient-reported outcomes in patients with ankylosing spondylitis (AS); however, there are limited data describing their use in real-world clinical practice. The objective of this analysis was to characterize and compare patients with AS who continued and discontinued their biologic therapy within 12 months of initiation in the US-based Corrona Psoriatic Arthritis/Spondyloarthritis (PsA/SpA) registry.
Methods: This descriptive analysis included all patients with AS aged ≥ 18 years enrolled in the Corrona PsA/SpA registry between March 2013 and March 2016 who received a biologic at the time of registry enrollment and had ≥ 1 follow-up visit. Patients were assigned to a cohort depending on their continued or discontinued use of their biologic agents at the first follow-up visit (mean [SD] follow-up, 8.8 [4.6] months). Patient demographics, clinical presentation, patient-reported outcomes and past/current treatments were assessed and compared between cohorts using t-tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. Reasons for discontinuation of the index biologic (e.g., side effects, social reasons, lack of effect, doing well or other) were also described in this study.
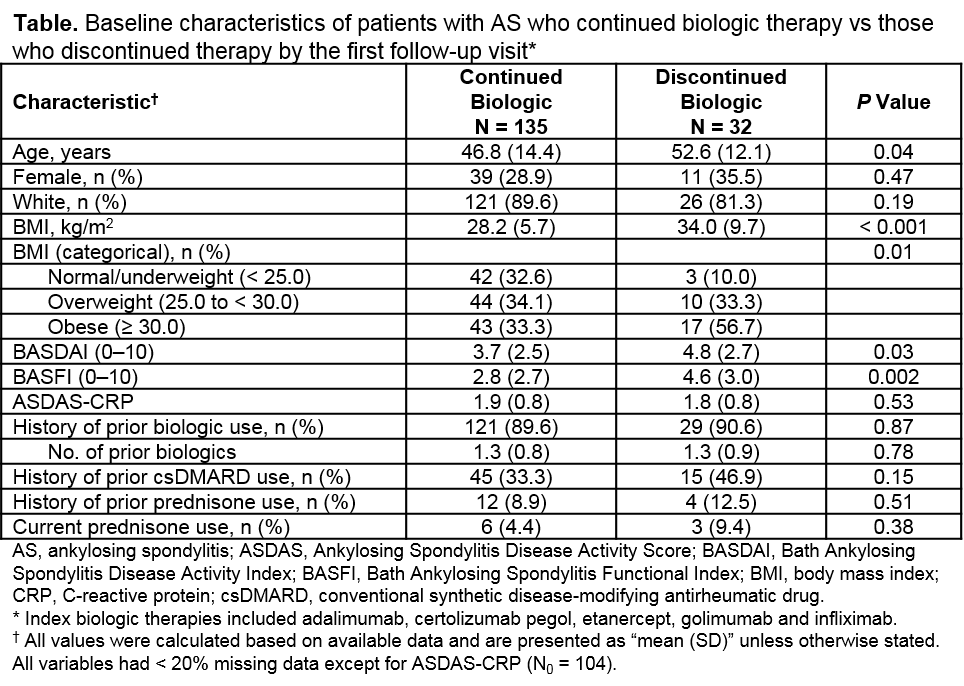
Results: Of the total of 167 patients with AS who met the inclusion criteria, 32 patients (19.2%) discontinued the index biologic therapy by the first follow-up visit, including 12 patients who switched to another biologic therapy. Baseline characteristics of patients who continued vs discontinued a biologic by their first follow-up visit were similar with regards to sex, race, education, insurance type and past/current treatments. However, patients who discontinued their index biologic were significantly older (52.6 vs 46.8 years); were more likely to be obese (56.7% vs 33.3%) with greater body mass index (34.0 vs 28.2 kg/m2); and had significantly worse Bath Ankylosing Spondylitis Disease Activity Index scores (4.8 vs 3.7) and Bath Ankylosing Spondylitis Functional Index scores (4.6 vs 2.8), but similar Ankylosing Spondylitis Disease Activity Scores (1.8 vs 1.9), at enrollment compared with patients who continued their biologic therapy (Table). Among the 9 patients (28.1%) who reported a reason for discontinuation, lack of effect (55.6%) was the most common reason, followed by other reasons (22.2%), side effects (11.1%) and social reasons (11.1%).
Conclusion: Results from the Corrona PsA/SpA registry demonstrated that patients with AS who discontinued their biologic therapy by the first follow-up visit were significantly older, more overweight and had worse patient-reported disease activity and function at enrollment compared to those who remained on the therapy. Lack of effect was the most common reason for biologic discontinuation.
To cite this abstract in AMA style:
Mease PJ, van der Heijde D, Karki C, Liu M, Pandurengan R, Park Y, Greenberg JD. Discontinuation of Biologic Therapy in Patients with Ankylosing Spondylitis—Data from the Corrona Psoriatic Arthritis/Spondyloarthritis (PsA/SpA) Registry [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/discontinuation-of-biologic-therapy-in-patients-with-ankylosing-spondylitis-data-from-the-corrona-psoriatic-arthritisspondyloarthritis-psaspa-registry/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-of-biologic-therapy-in-patients-with-ankylosing-spondylitis-data-from-the-corrona-psoriatic-arthritisspondyloarthritis-psaspa-registry/