Session Information
Date: Monday, November 18, 2024
Title: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Roughly 30,000 people are living with osteogenesis imperfecta (OI) in the US, most of them adults. Fractures rates are high in OI adults with minimal information about their use of anti-fracture drugs. This retrospective observational study used US-based claims data to describe anti-fracture drug use, disability, and functional limitations (proxied by wheelchair and other durable medical equipment DME use) in a large cohort of OI adults.
Methods: Individuals with OI were identified by International Classification of Diseases, 9th and/or 10th (ICD-9: 756.5; ICD-10: Q78.0) codes from a 5% random sample US Medicare Fee-for-Service data between 2006-2021 and MarketScan claims data, 2006-2022. To increase the validity of the OI diagnosis, we required one inpatient hospital ICD-9/10 code or at ≥2 outpatient ICD-9/10 codes for OI. We included patients who were age 18+ with ≥12 months of continuous coverage before or after the index date and continuous coverage during follow-up. We categorized patient age and identified those with more severe OI as using a manual or power wheelchair based on DME claims. Anti-fracture drug prescription records were obtained from pharmacy and medical procedure claims. Therapies included oral and IV bisphosphonates, PTH-analogs, denosumab and romosozumab. Drug use was defined as ever receiving a prescription.
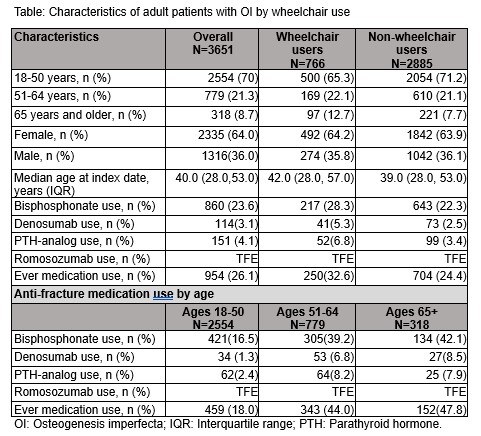
Results: The cohort included 3651 adults with OI(Table). Most were <50 years in both wheelchair and non-wheelchair groups. In the Medicare cohort, 59% of patients were < 65 years of age with disability as the reason for initial Medicare entitlement. Overall, 21% of the cohort were wheelchair users. There were more females in the cohort, and the percentage of females was similar between wheelchair and non-wheelchair users. The proportions of medication use were low (26.1% overall) in both wheelchair and non-wheelchair groups and varied with age (18.0% in adults younger than 50, rising to 47.8% in those age 65+). In all age groups, the most commonly used drug was a bisphosphonate (22-28%). 2.5-5% used denosumab and 3-7% used a PTH analog. The prevalence of medication use increased with age; but, the portion of anti-fracture drug use in the older age groups remained below 50%.
Conclusion: In a large cohort of adults with OI with commercial insurance or Medicare, disability and wheelchair use were common. Anti-fracture drug use increased with age; but, the prevalence of medication use remained low in adults with both mild and severe OI phenotypes. These data provide a unique understanding of anti-fracture drug utilization in adults with OI. Additional analyses will provide information about health care use in OI adults and inform crucial healthcare resources.
To cite this abstract in AMA style:
liu W, Curtis J, Folkestad L, Holladay E, Liu Y, Xie F, Zhang J, Orwoll E. Disability, Functional Limitation, and Use of Anti-fracture Drug Therapies in a Cohort of 3651 U.S. Adults with Osteogenesis Imperfecta [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/disability-functional-limitation-and-use-of-anti-fracture-drug-therapies-in-a-cohort-of-3651-u-s-adults-with-osteogenesis-imperfecta/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disability-functional-limitation-and-use-of-anti-fracture-drug-therapies-in-a-cohort-of-3651-u-s-adults-with-osteogenesis-imperfecta/