Session Information
Date: Monday, November 11, 2019
Title: 4M125: Pediatric Rheumatology: Outcomes & Quality of Life (1920–1925)
Session Type: ACR/ARP Abstract Session
Session Time: 4:30PM-6:00PM
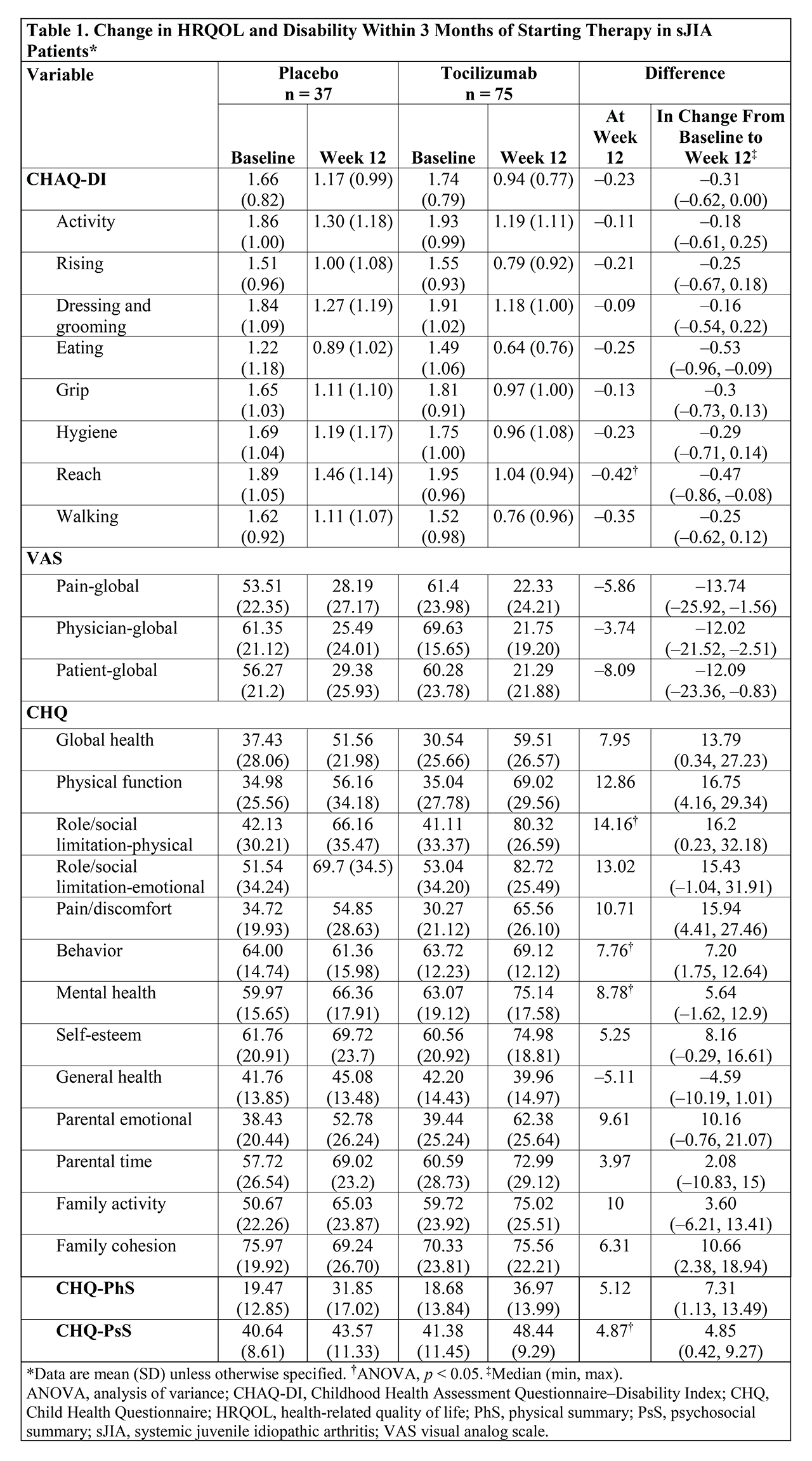
Background/Purpose: Tocilizumab (TCZ) was approved for the treatment of systemic juvenile idiopathic arthritis (sJIA) and polyarticular juvenile idiopathic arthritis (pJIA) based on the results of 2 large phase 3 clinical trials.1,2 Physical function, measured by the Childhood Health Assessment Questionnaire–Disability Index (CHAQ-DI), and health-related quality of life (HRQOL), measured by the Child Health Questionnaire (CHQ), were evaluated in both trials. The objective of this post hoc analysis of data from both phase 3 trials of TCZ was to examine measures of disability and HRQOL in patients with sJIA or pJIA treated with TCZ for up to 2 years.
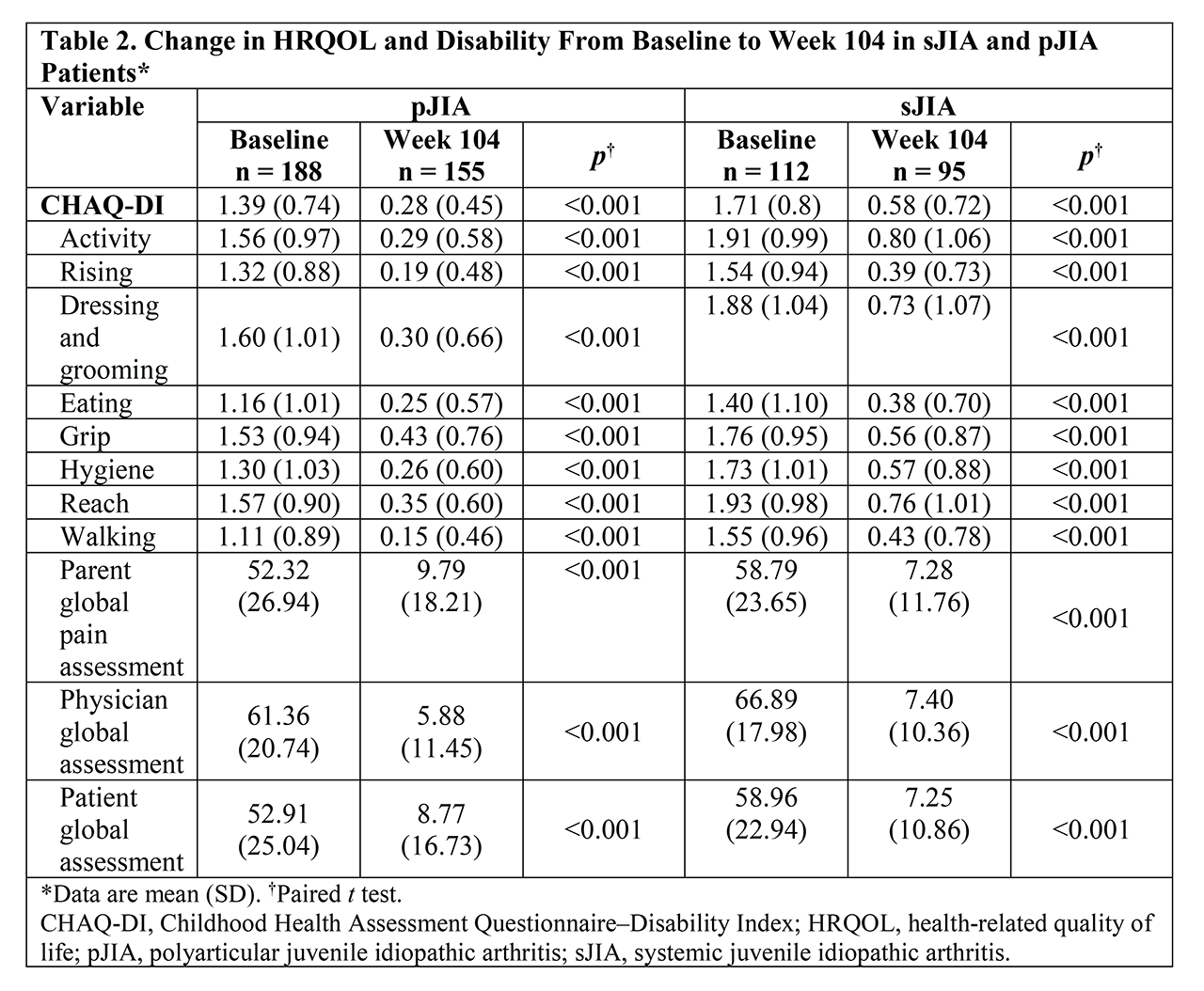
Methods: Eligible patients aged 2 to 17 years of age diagnosed with active (≥6 months) sJIA or pJIA according to International League of Associations for Rheumatology criteria received TCZ IV or placebo every 4 weeks (pJIA) or every 12 weeks (sJIA). For sJIA patients, changes within 3 months of treatment initiation with TCZ (baseline) were compared between TCZ- and placebo (PBO)-treated patients using CHAQ-DI, pain-global assessment, physician (MD) global assessment, and patient global assessment using analysis of variance adjusted for treatment group. Changes in CHAQ-DI overall and domain scores from baseline to 2 years were compared for sJIA and pJIA patients treated with TCZ using the unpaired t test; similarly, for sJIA patients treated with TCZ, changes in CHQ domain and summary scores between baseline and 2 years were assessed.
Results: sJIA patients experienced clinically relevant improvement of physical function (CHAQ-DI) and reduction in pain (pain-global). Mean (SD) CHAQ-DI scores for patients treated with PBO and TCZ were 1.7 (0.8) for both groups at baseline and 1.2 (1.0) and 0.9 (0.8), respectively, at week 12 (week 12 mean difference, –0.2; median [range] change from baseline to week 12 difference, –0.3 [–0.6 to 0.0]). sJIA patients treated with TCZ had significantly improved socialization, behavior, mental health, and CHQ-psychosocial summary scores after 3 months compared with those receiving PBO (Table 1). Marked improvement in all CHAQ-DI domains over 2 years was observed with TCZ treatment in both sJIA and pJIA patients (Table 2); improvement rates in patient well-being (patient-global) were 87.7% in sJIA patients and 83.4% in pJIA patients. There was also significant improvement (p < 0.05) in most domains of HRQOL (CHQ-domain scores) in patients with sJIA; of note, sJIA patients experienced marked improvement in mean scores from baseline to week 104 for pain/discomfort (31.7 to 75.3), self-esteem (61.0 to 76.0), mental health (62.1 to 76.8), and social limitation-emotional (52.6 to 86.2).
Conclusion: TCZ treatment resulted in statistically significant and clinically relevant improvements in function and HRQOL in patients with sJIA or pJIA over 2 years.
References: 1. De Benedetti F et al. N Engl J Med 2012;367:2385-95. 2. Brunner HI et al. Ann Rheum Dis 2015;74;1110-7.
To cite this abstract in AMA style:
Brunner H, Chen C, Martini A, Espada G, Joos R, Akikusa J, Chaitow J, Gámir Gámir M, Kimura Y, Rietschel C, Siri D, Smolewska E, Schmeling H, Brown D, De Benedetti F, Lovell D, Huang B, Ruperto N. Disability and Health-Related Quality of Life Outcomes in Patients with Systemic or Polyarticular Juvenile Idiopathic Arthritis Treated with Tocilizumab in Randomized Controlled Phase 3 Trials [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/disability-and-health-related-quality-of-life-outcomes-in-patients-with-systemic-or-polyarticular-juvenile-idiopathic-arthritis-treated-with-tocilizumab-in-randomized-controlled-phase-3-trials/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disability-and-health-related-quality-of-life-outcomes-in-patients-with-systemic-or-polyarticular-juvenile-idiopathic-arthritis-treated-with-tocilizumab-in-randomized-controlled-phase-3-trials/