Session Information
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) affects over 1 million Medicare enrollees. Despite the size of this population, costs for RA within Medicare remain poorly understood, especially since biologic DMARDs (bDMARDs) have become an important part of care for RA. Greater insight into the detailed cost to Medicare for patients with RA would be valuable to both policy makers and health care providers, supporting planning for the allocation of limited resources.
Methods: Subjects were Medicare beneficiaries enrolled in the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS). Patient inclusion criteria were age greater than 18 years, DAS28-CRP recorded in the BRASS registry in 2006 or later, and at least 18 consecutive months of available Medicare claims data including prescription drug utilization. Cost and utilization data for inpatient admissions, outpatient care, and physician services were acquired from the Medicare Standard Analytics Files for 2006-2010. Prescription drug utilization data was obtained from the Prescription Drug Event file, reflecting Medicare Part D claims. Prescription medication costs were calculated by multiplying utilization by a standardized per unit price for each unique National Drug Code, determined using the Centers for Medicare and Medicaid Services’ (CMS) National Average Drug Acquisition Cost (NADAC) Tool or from the lowest per unit price listed on the drugs.com website (if no NADAC cost was available). RA-specific costs were determined by isolating claims with an RA-related ICD-9-CM diagnosis code. RA-specific prescription costs were isolated using a comprehensive list of RA-related medications. Each calendar year in which a patient had Medicare utilization was treated as an independent observation, referred to as a patient-year. All costs were converted to 2015 dollars using the consumer price index medical care component.
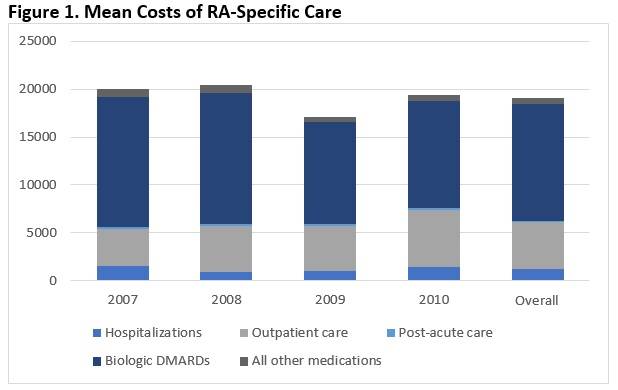
Results: 197 individual patients met inclusion criteria, contributing a total of 435 patient years. Mean annual direct cost of all medical care was $36,643 (95% CI 33,603-39,682). RA-specific care accounted for $19,216, or 52% of total costs. Within RA-specific care, hospitalizations accounted for 6.3% of costs, outpatient care 25.6%, medications 67.3%, and post-acute care 0.9% (see Figure 1). There was no significant difference in distribution of RA-specific costs across years (p = 0.37). RA-specific costs were dominated by costs of bDMARDs. The mean annual cost for bDMARDs across the total cohort (bDMARD users and non-users) was $12,167 (95% CI 10,357-13,976), representing 94.6% of RA-related drug costs and 63.6% of total RA-specific costs.
Conclusion: While bDMARDs have expanded treatment options for RA patients, the cost of bDMARDs is the primary driving factor for the cost of RA-specific care.
To cite this abstract in AMA style:
Hresko A, Zhang Z, Colls J, Weinblatt ME, Shadick NA, Solomon D. Direct Medical Costs for Medicare Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/direct-medical-costs-for-medicare-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/direct-medical-costs-for-medicare-patients-with-rheumatoid-arthritis/