Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: We reported partial direct healthcare costs associated with damage accrual in the SLICC Inception Cohort (Barber M. Arthritis Care Res 2020;72:1800). We have supplemented this data by querying a cohort subset on all healthcare use and lost time in paid/unpaid labour and now provide complete direct and indirect costs (DC, IDC) for the full cohort, stratified by damage.
Methods: Between 1999 and 2011, SLE patients from 31 centres in 10 countries were enrolled in the SLICC Inception Cohort within 15 months of diagnosis. Damage (SLICC/ACR Damage Index [SDI]) and limited healthcare use (hospitalizations, medications, and dialysis) were collected annually to July 2022. Starting in 2015, 18 sites collected supplemental economic data annually (physicians, non-physician healthcare professionals, emergency room, laboratory tests, radiological/other procedures, outpatient surgeries, help obtaining medical care, and lost time in paid/unpaid labour).DC were calculated by multiplying each health resource by its corresponding 2024 Canadian unit cost. Total IDC included: 1) absenteeism (time lost from paid labour because of illness), 2) presenteeism (degree of patient self-reported productivity impairment in paid/unpaid labour, based on a visual analogue scale), and 3) opportunity costs (additional time patients would be working in paid/unpaid labour if not ill). Opportunity costs were calculated as the difference between time patients reported working versus that worked by an age, gender, and geographic-matched general population in paid/unpaid labour. IDC from paid/unpaid labour were valued using age-and-gender-specific wages from Statistics Canada. Multiple imputation was used to predict missing costs for patients in the full cohort who provided only limited economic data for all observations. At each assessment, patients were assigned to one of six damage states (SDI = 0, 1, 2, 3, 4, >= 5) and annual costs, both unimputed and including imputations, were stratified by SDI. Means and 95% CIs were compared.
Results: 1694 patients (88.8% female, 48.9% White, mean age at diagnosis 34.6 years) were followed a mean of 10.5 years. Of these 1694 patients, 766 (89.7% female, 41.4% White, mean age at diagnosis 33.0 years) completed the supplemental economic questionnaire (Table 1). Their mean disease duration at the time of this questionnaire was 10.9 years and this subset provided economic data a mean of 3.5 years. Among the subset completing the supplemental questionnaire, on average, IDC, primarily from unpaid labour, accounted for 81.2% of total costs (Table 2). For the full cohort, annual DC and IDC increased with increasing SDI (SDI = 0: total costs $35,417 [95%CI $32,563, $38,271]; SDI >= 5: total costs $95,149 [95%CI $86,178, $104,119]) (Figure 1).
Conclusion: Patients with the highest versus the lowest SDI incurred complete DC that were 6.0-fold and IDC 2.1-fold higher. Even patients with no or minimal damage still experienced considerable IDC. IDC exceeded DC, on average, by 4.6-fold. Novel/emerging biologics, which potentially attenuate damage accrual, may be associated with substantial cost savings, particularly if IDC, which are a considerable portion of total costs, are incorporated in the economic analysis.
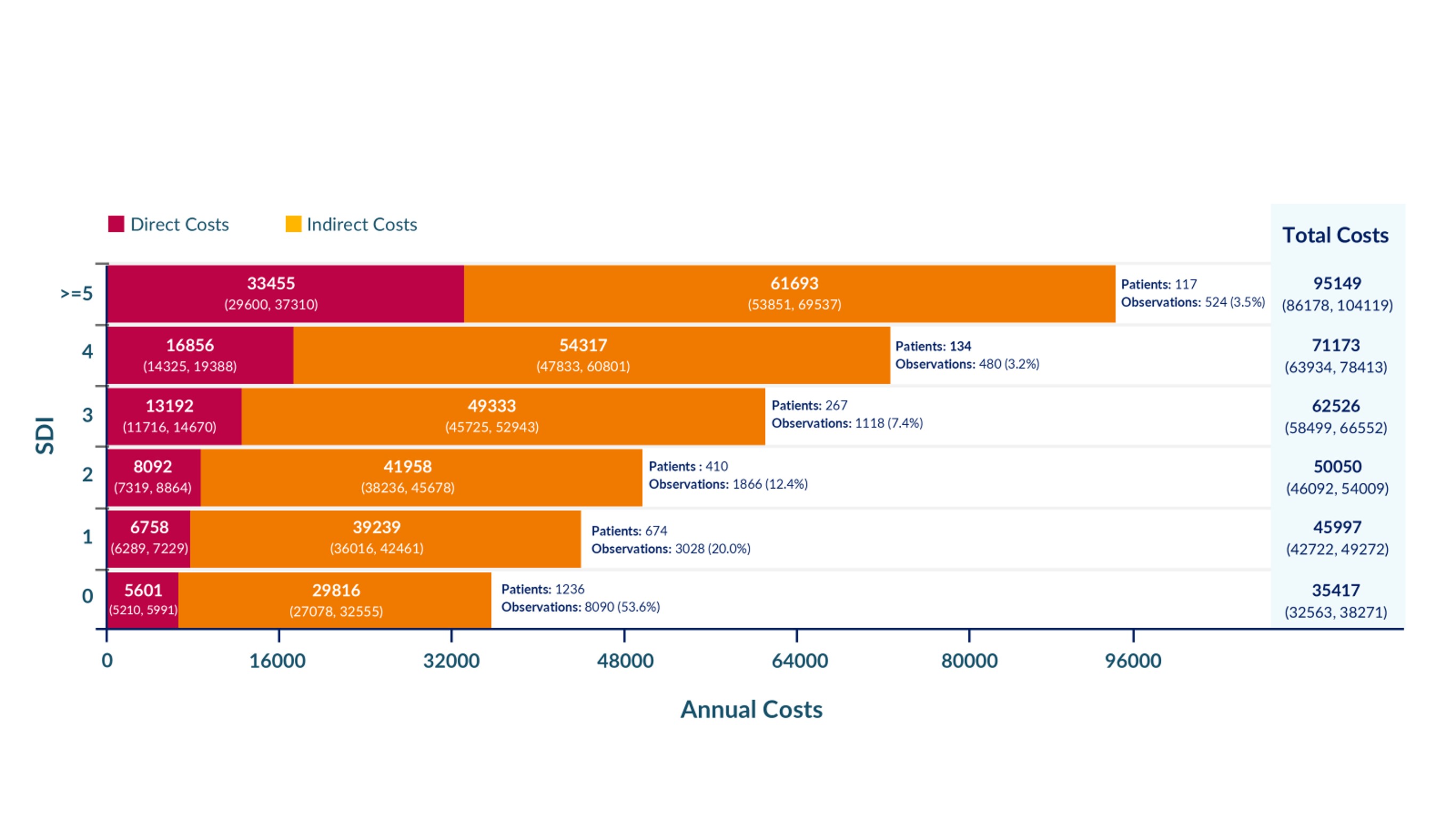 Table 1. Participant Characteristics
Table 1. Participant Characteristics
.jpg) Table 2. Annual complete direct, indirect, and total costs (in 2024 Canadian dollars) for the cohort subset providing complete cost data, stratified by SDI (n = 2414 observations). Values are means.
Table 2. Annual complete direct, indirect, and total costs (in 2024 Canadian dollars) for the cohort subset providing complete cost data, stratified by SDI (n = 2414 observations). Values are means.
.jpg) Figure 1. Annual imputed complete direct, indirect, and total costs (in 2024 Canadian dollars) for the full cohort, stratified by SDI (n = 15,106 observations)
Figure 1. Annual imputed complete direct, indirect, and total costs (in 2024 Canadian dollars) for the full cohort, stratified by SDI (n = 15,106 observations)
To cite this abstract in AMA style:
Barber M, Hanly J, Urowitz M, Bruce I, St-Pierre Y, Gordon C, Bae S, Romero-Diaz J, Sanchez-Guerrero F, Bernatsky S, Wallace D, A. Isenberg D, Rahman A, Merrill J, Fortin P, Gladman D, Petri M, Ginzler E, Dooley M, Ramsey-Goldman R, Manzi S, Jönsen A, Alarcón G, van Vollenhoven R, Aranow C, mackay M, Ruiz-Irastorza G, Lim S, Inanç M, Kalunian K, Jacobsen S, Peschken C, Kamen D, Askanase A, Clarke A. Direct and Indirect Costs Associated with Damage Accrual: Results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/direct-and-indirect-costs-associated-with-damage-accrual-results-from-the-systemic-lupus-international-collaborating-clinics-slicc-inception-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/direct-and-indirect-costs-associated-with-damage-accrual-results-from-the-systemic-lupus-international-collaborating-clinics-slicc-inception-cohort/
