Background/Purpose: Difficult-to-treat (DTT) group in the MOTION study included symptomatic refractory gouty arthritis (RGA) patients with ≥3 flares, refractory to NSAIDs/colchicine/steroids or to uric acid lowering therapy (ULT) due to contraindication, intolerance, or lack of efficacy. There is lack of evidence on burden of illness in DTT patients. The study assessed burden of illness in DTT patients over 1 year.
Methods: A post hoc descriptive comparison was done between DTT vs the other refractory gout (ORG) patients using data from the MOTION (1 year, multinational, non-interventional, prospective, observational) study. Among DTT patients, outcomes for patients with tophi at baseline and patients who discontinued ULT prior to study entry were also summarized. The study outcomes (evaluated at baseline, by time point and pooled yearly) included health status using EuroQol Group 5‑Dimension (EQ-5D) questionnaire, the Gouty arthritis Assessment Questionnaire–Gouty arthritis Impact Scale (GAQ‑GIS), pain assessment, healthcare utilization over 1 year and lost work productivity. Patient and physician satisfaction with treatment were measured by the Patient Global Assessment of Response to Treatment and Investigator Global Assessment of Response to Treatment. Continuous and categorical variables were reported as (mean ± SD) and as proportions, respectively, for the DTT and ORG patient groups.
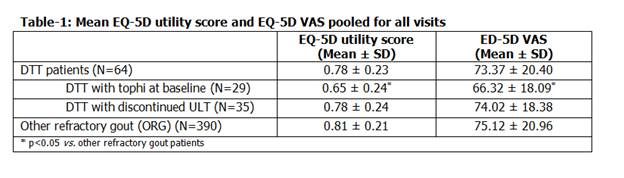
Results: Among 454 patients enrolled in the MOTION study, 64 DTT patients (tophaceous group=29, discontinued ULT=35) were included in the analysis (mean age 55.2, males 85.9%). DTT patients had lower mean EQ-5D utility score and EQ-5D VAS score compared to ORG group, with worse scores in the tophaceous group (Table 1). Tophaceous group patients experienced greater difficulty on all EQ-5D dimensions and pain/discomfort was the most affected dimension in all groups. DTT patients expressed greater overall gouty arthritis concern compared to ORG patients (76.5 vs 72.4%), with highest score in the tophaceous group (85.5%) for pooled data. Tophaceous group reported a higher score on all five dimensions of GAQ GIS. About 45.3% of DTT patients reported severe pain during last flare vs 37.2% ORG patients. Healthcare utilization was greater among DTT than ORG patients (35.9 vs 21.1%). A higher proportion in the tophaceous group visited emergency unit (55.2%), hospital (41.4%) and doctors (89.7%) over one year. Physicians and patients in DTT group were more likely to report poor response to treatment compared to ORG group. DTT group missed 23.4% of their working days compared to 15.6% in ORG patients at baseline, the highest being the tophaceous group (31%).
Conclusion: DTT patients had worse health status and reported higher resource utilization with significantly poorer outcomes reported in tophaceous gout patients, suggesting a high unmet medical need in such patients.
Disclosure:
L. Bessette,
Novartis,
2;
F. Lioté,
Novartis, Ipsen, Sanofi,
1,
Novartis, SOBI, Astra-Zeneca, Savient, Ipsen, Menarini, Mayoly-Spindler ,
2,
Novartis, Ipsen, Menarini, Savient, Astra-Seneca, Mayoly-Spindler,
5;
C. Moragues,
Novartis,
2;
R. Moericke,
Novartis,
2;
Z. Zhiyi,
Novartis,
2;
A. Ferreira,
Novartis Pharma AG, Basel,
3;
P. Lecomte,
Novartis Pharma AG, Basel,
3;
S. Kessabi,
Novartis Pharma AG, Basel,
3;
H. Tian,
Novartis Pharmaceuticals Corporation, East Hanover NJ,
3;
J. A. Singh,
Takeda, Savient, Novartis ,
2,
Savient, Takeda, Regeneron and Allergan,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/difficult-to-treat-gouty-arthritis-associated-with-poor-health-related-quality-of-life-and-high-resource-utilization-post-hoc-analysis/