Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Although SLE disproportionately affects minority racial groups, this population is significantly under-represented in clinical trials, increasing risk for underpowered, incorrect conclusions in race-based sub-group analyses. The decision to participate in clinical research is complex. Primary care providers (PCPs) have the ability to inform their patients about research and refer to specialists who participate in clinical trials. We evaluated knowledge of SLE and thoughts about clinical research participation with both PCPs and lupus patients.
Methods: Lupus patients and PCPs completed a pre-test consisting of knowledge and belief questions prior to engaging in an educational program about lupus, clinical research, and human subjects’ protections. As part of the post-test, the same set of questions were repeated. Knowledge questions were analyzed by Fisher’s exact test or McNemar’s test for between group and within group comparisons. Belief questions (Likert scale ratings) were analyzed by Mann-Whitney or Wilcoxon matched pairs for between group and within group comparisons.
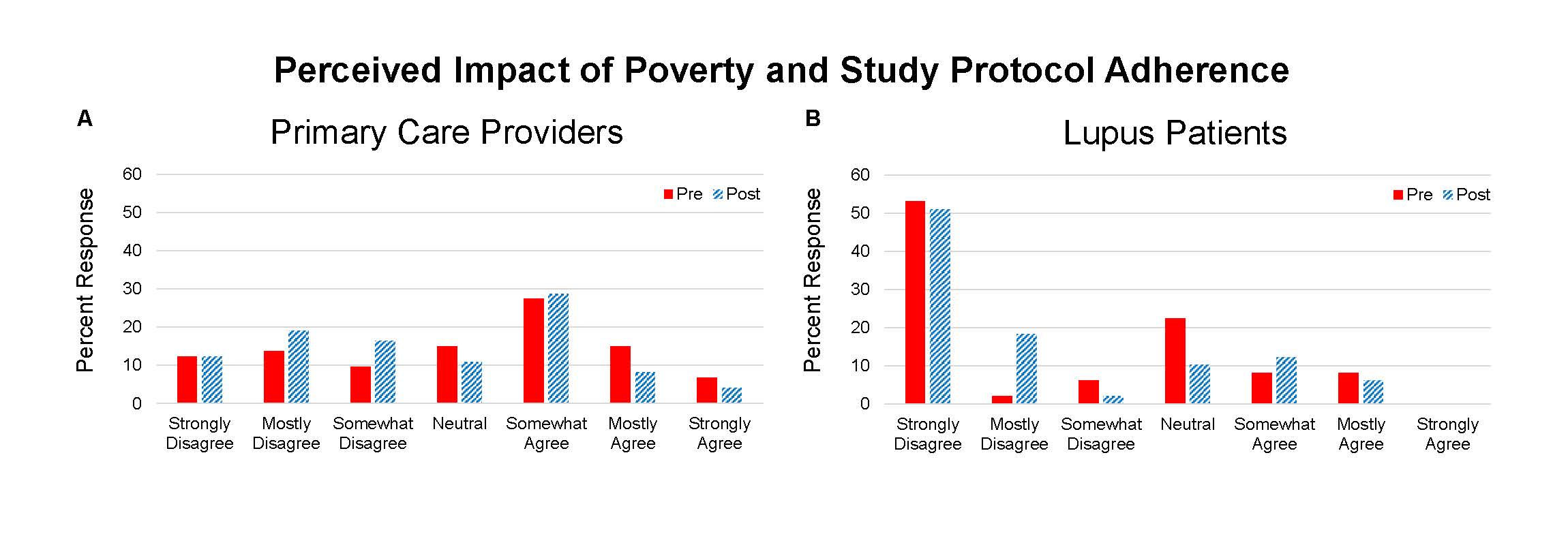
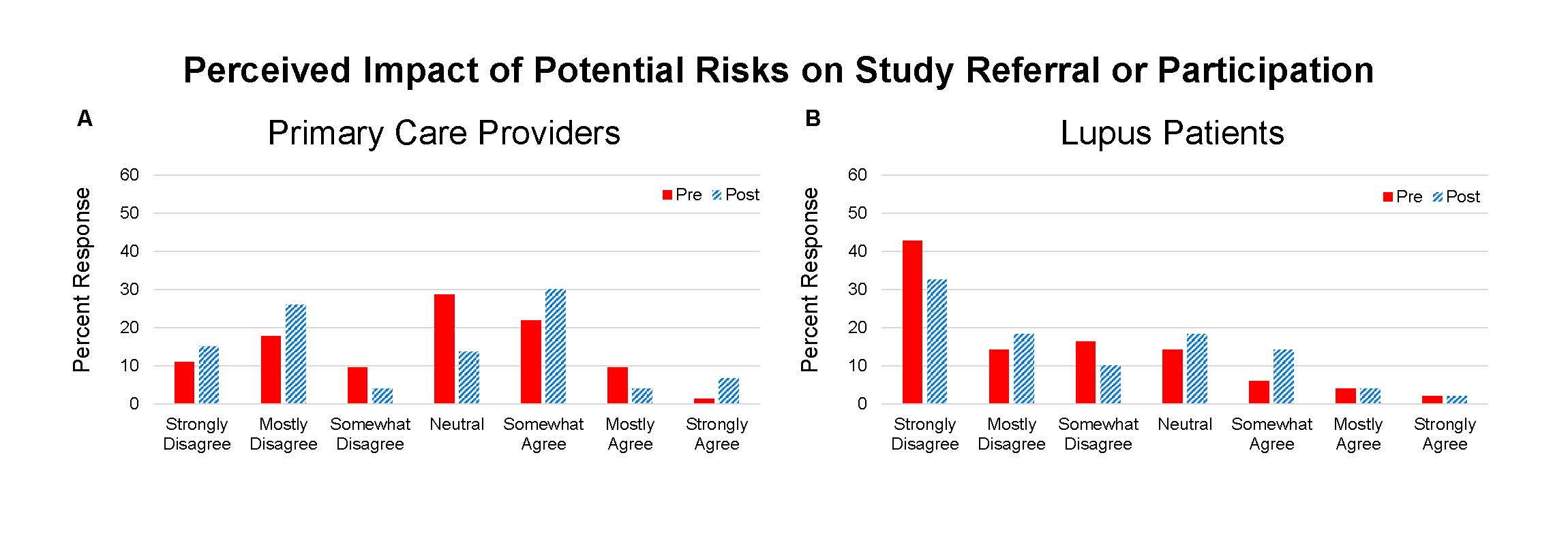
Results: 73 providers and 49 lupus patients completed the questionnaires and program. Knowledge topics included 1) triggers of SLE, 2) racial differences in lupus, and 3) elements of informed consent. On the pre-test there were differences between groups in scoring of the informed consent question (PCP 82% correct and patient 49%, p=0.0001). On the post-test the patients’ scores improved (PCP 92% and patient 73%, p=0.0098). The education program resulted in improvement in all knowledge scores for PCPs and patients (Table 1). Points of view about clinical trials included questions about the impact of differing racial background between provider and patient, education level of study participants, risks of trial participation, and effect of poverty on protocol compliance – the latter two resulted in the greatest incongruence (Figure 1 and 2).
Conclusion: Concepts about race, education, and poverty may impact interactions between clinicians and patients that could inhibit referral to clinical trial centers and clinical trial participation. We found that some providers may hold a belief that indigent patients are poor candidates for clinical trials. Despite a shift away from this opinion following an educational program, providers remained much more likely to retain this point of view compared to lupus patients. Further effort to optimize conditions for clinical trial access to all patients is needed. This work may benefit from better understanding of the barriers that have led to underrepresentation of minority patients.
To cite this abstract in AMA style:
Arriens C, Forciea D, James J, Merrill J. Differing Opinions on Clinical Research Between Healthcare Providers and Lupus Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/differing-opinions-on-clinical-research-between-healthcare-providers-and-lupus-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/differing-opinions-on-clinical-research-between-healthcare-providers-and-lupus-patients/