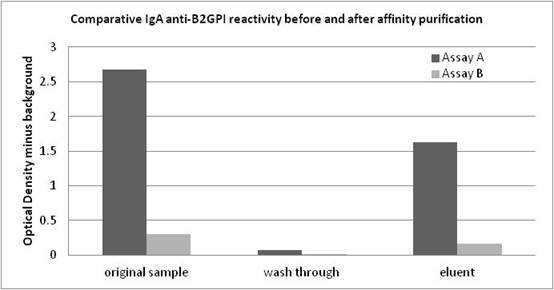
Background/Purpose: IgA anti-β2Glycoprotein I (aβ2GPI) antibodies remain controversial in the assessment of thrombotic risk in spite of several studies indicating an association with thromboembolic events in SLE and antiphospholipid syndrome (APS) patients. In addition, recent proficiency testing revealed widely discrepant results between 2 commonly used IgA aβ2GPI ELISAs on SLE and catastrophic APS samples. This controversy may have contributed to exclusion of IgA aCL and aβ2GPI antibodies from the current classification criteria for APS. One hypothesis is that coated β2GPI of one assay displayed the open (reactive) β2GPI configuration while the other had the closed (non-reactive) configuration. Methods: Four sera selected from positive SLE and APS patients having discrepant IgA aβ2GPI reactivity; strongly positive in assay A (144-388 A units) and negative in assay B (9.9-18.6 A units). Cut-off was <20 A units in both assays. These samples were also strong IgG aβ2GPI (143-237 G units in assay A). A negative serum was used as control. IgA antibodies were affinity purified (Peptide M, Invivogen, Inc) to investigate β2GPI reactivity. Column wash-through and eluents were tested on both IgA aβ2GPI assays. Results were normalized to total protein. To determine the nature of differential reactivity, assay conjugates and controls/calibrators from assay A and B were interchanged.< Results: IgA eluents from IgA aβ2GPI positive samples reacted 10x stronger on assay A compared to assay B (graph). ODs of assay B were within the negative range (<0.200). When normalized to protein content, the eluents showed no cross-reactivity for IgG or IgM aβ2GPI antibodies confirming IgA isotype specificity. IgG aβ2GPI antibodies were detected in the wash-through. Conjugate from assay A reacts with reagents from both assays. However, conjugate from assay B reacts only with assay B reagents. This confirms that β2GPI-coated plates of assay B bind IgA aβ2GPI antibodies, questioning the open/closed hypothesis. When both conjugates were compared in assay B, conjugate from assay A was 4x more reactive, suggesting that the ability of assay A to detect IgA aβ2GPI antibodies is partially dependent on the anti-IgA conjugate and calibration. Conclusion: These results confirm not only the presence of IgA aβ2GPI antibodies in the selected patient samples but highlight an IgA conjugate reactivity issue for assay B, causing an underestimation of IgA aβ2GPI. This finding may assist in ongoing standardization efforts of APS antibody testing. In addition, conclusions from published clinical studies may need to be revised as some assays may understate IgA significance.
Disclosure:
D. B. Hood,
Corgenix, Inc.,
3;
K. R. Snyder,
Corgenix, Inc.,
3;
T. R. Buckner,
Corgenix, Inc.,
3;
B. L. Hurley,
Corgenix, Inc.,
3;
K. R. Pitts,
Corgenix, Inc.,
3;
L. R. Lopez,
Corgenix, Inc.,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/differential-assay-reactivity-of-iga-anti-b2glycoprotein-i-antibodies-implications-for-clinical-interpretation-of-antiphospholipid-antibody-testing/