Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The wish of a treating physician is to make sure, that the patient gets an effective and safe therapy and is satisfied with the treatment. The question is how to measure the patient’s satisfaction with the therapy.
SUSTAIN is a prospective, multi-center non-interventional study in Germany to observe long term efficacy and safety, quality of life and other patient reported outcomes (PROs) in patients with active psoriatic arthritis (PsA) under treatment with ustekinumab in routine clinical care. In this ongoing study, up to 400 patients were planned to be included at 75 centers for 160 weeks with visit intervals at week 0 and 4 and then every 12 weeks of treatment with ustekinumab according to the label. In this non-interventional study, all patients were diagnosed with psoriatic arthritis according to the CASPAR Criteria.
Here we examine the correlation between treatment satisfaction of the patients with satisfaction of the physicians. Furthermore, we correlate satisfaction with the effectiveness of the therapy for both patients and physicians, regarding the joints and the skin.
Methods: In this interim analysis, results of baseline data of 336 patients and of the documented visits up to week 112 were evaluated. Reported outcomes on satisfaction of patients and physicians with effectiveness and tolerability of therapy were assessed and correlated with disease severity measures such as number of swollen and tender joints and psoriasis affected body surface area (BSA).
The correlation between measured variables was assessed using Spearman’s Rank Correlation Coefficient (CC). With a possible range from -1 to +1, a correlation coefficient between -0.3 and 0.3 was defined as no /weak correlation, between -0.3 and -0.7/ 0.3 and 0.7 as moderate correlation, and values below -0.7 or above 0.7 as strong correlation.
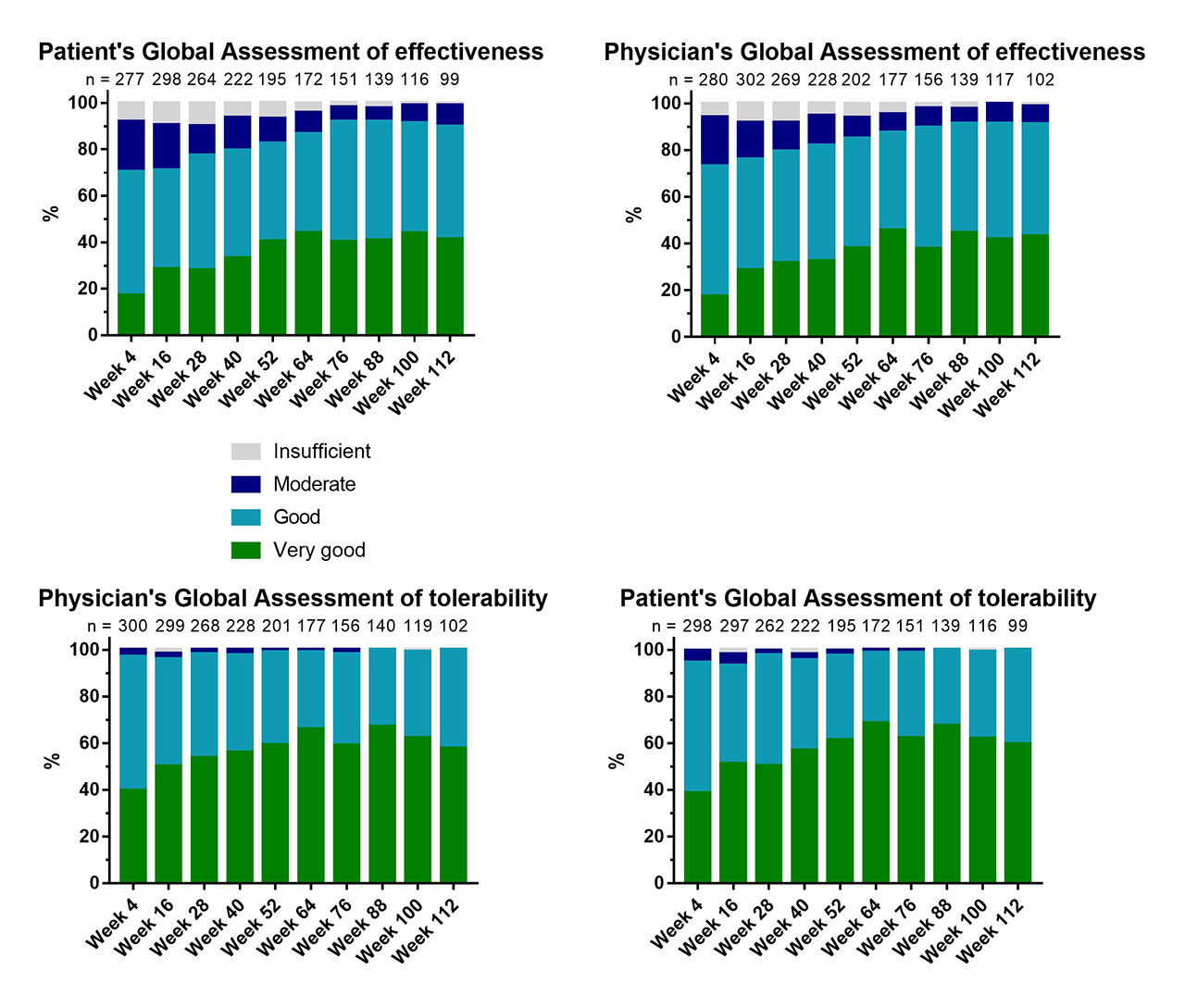
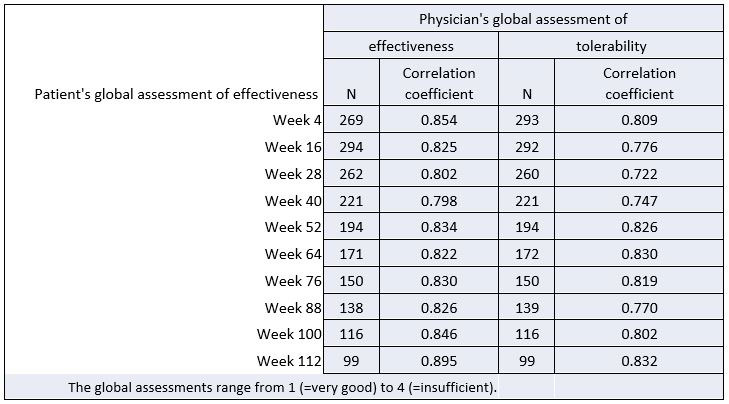
Results: The patients’ and physicians’ global assessments of effectiveness and tolerability were evaluated at every 12 weeks of treatment (Figure 1). The CCs between patient’s and physician’s assessments over 112 weeks of treatment were in the range of 0.798 and 0.895 for effectiveness and 0.722 and 0.832 for tolerability (Table 1). Therefore, the CCs for global assessments can be classified as strong.
The CCs between physicians’ and patients’ assessments of effectiveness for tender joint count, based on 78 joints at week 112, were 0.321 (n=96) and 0.247 (n=94) respectively. The CCs between physicians’ and patients’ assessments of effectiveness for swollen joint count based on 76 joints also at week 112 of treatment, were 0.232 (n=96) and 0.193 (n=94) respectively. The CCs between physicians’ and patients’ assessments of effectiveness for BSA were 0.227 (n=86) and 0.216 (n=85) respectively. Thus, the correlation between patients’ and physicians’ satisfaction with the effectiveness of the therapy can mostly be classified as weak.
Conclusion: The results showed strong correlation between patients’ and physicians’ global assessments of both effectiveness and tolerability with high treatment satisfaction. However, the PROs correlate weakly to moderately with disease measures of tender and swollen joint counts and BSA.
To cite this abstract in AMA style:
Wendler J, Wagener P, Ilka S, Movshovich E, Damann N, Behrens F. Differences in Correlation Between Objective Disease Measurements and Patient’s/physician’s Global Assessment in the Large Non-interventional Study SUSTAIN [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/differences-in-correlation-between-objective-disease-measurements-and-patients-physicians-global-assessment-in-the-large-non-interventional-study-sustain/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/differences-in-correlation-between-objective-disease-measurements-and-patients-physicians-global-assessment-in-the-large-non-interventional-study-sustain/