Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: National treatment recommendations are often used to optimize patient care and may differ from international recommendations. The aim of this study was to assess differences and similarities between the EULAR and ASAS-EULAR recommendations for the treatment of patients with PsA and axSpA, respectively, versus national PsA and axSpA treatment recommendations across Europe.
Methods: Rheumatologists from 15 European countries (Czech Republic, Denmark, Estonia, Finland, Iceland, Italy, Netherlands, Norway, Portugal, Romania, Slovenia, Spain, Sweden, Switzerland, and the United Kingdom) compared the most recent national treatment recommendations for PsA and axSpA with the “EULAR recommendations for the management of PsA with pharmacological therapies: 2019 update”1 and the “2016 update of the ASAS-EULAR recommendations for axSpA”2, in an online survey conducted between October 2021 and April 2022. The study was an initiative of the European Spondyloarthritis Research Collaboration Network (EuroSpA RCN).3
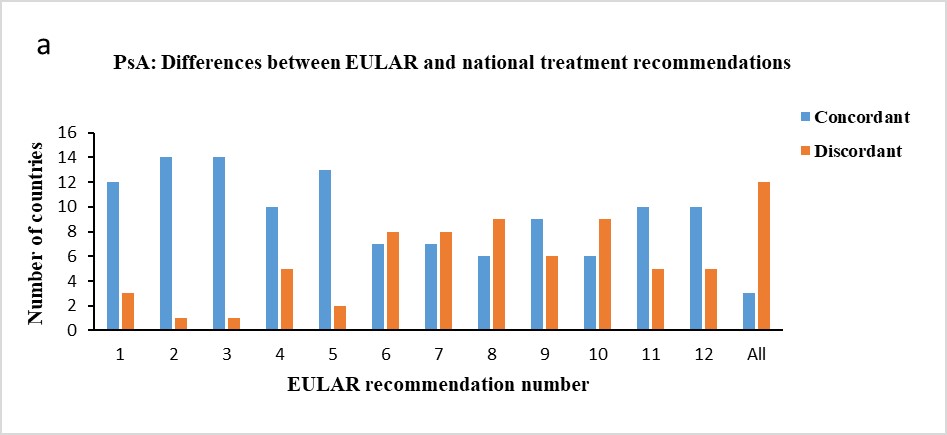
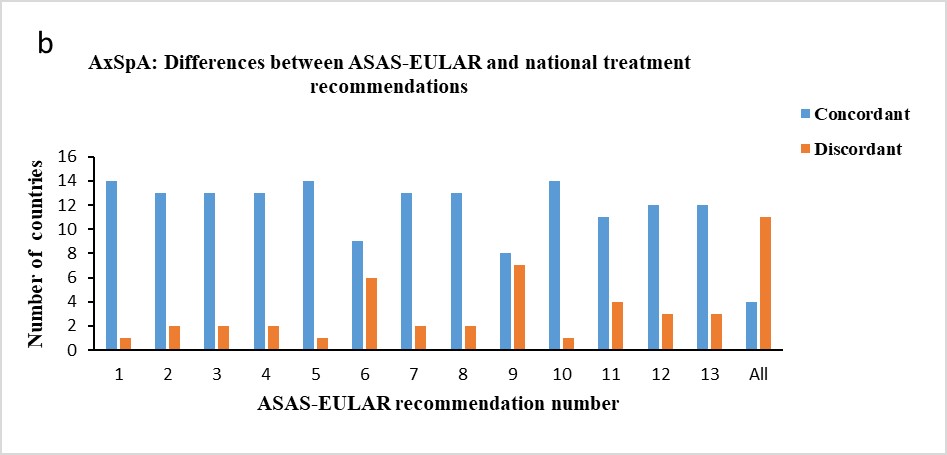
Results: Three countries (Czech Republic, Netherlands, and Spain) followed all EULAR recommendations for treating patients with PsA and four countries (Czech Republic, Italy, Spain, and Switzerland) all ASAS-EULAR recommendations for axSpA. Five countries had no national treatment recommendations for PsA and/or axSpA, but had other rules or regulations to follow, for which the comparisons in this study were performed. In six countries, the national treatment recommendations for PsA predated the 2019 EULAR recommendations and in one country the national treatment recommendations for axSpA predated the 2016 ASAS-EULAR recommendations. More differences were seen between the EULAR and the national treatment recommendations for PsA than between the ASAS-EULAR and the national treatment recommendations for axSpA (Figure 1a,b).
Discrepancies between international and national treatment recommendations included: Entry criteria for start of a biologic/targeted synthetic disease-modifying anti-rheumatic drug (b/tsDMARD) varied and were the most stringent in Romania, where DAPSA >28 for PsA and BASDAI >6 and ASDAS ≥2.5 for axSpA were required for the start of a bDMARD. Regarding PsA, in two countries (Finland and Switzerland) a conventional synthetic DMARD should be initiated before a b/tsDMARD including in patients with predominantly enthesal or axial disease. In several countries, no preference for interleukin-17 inhibitors was given for PsA patients with significant skin involvement. The positioning of Janus Kinase inhibitors (JAKi) differed across countries, e.g. in Estonia JAKi were indicated after failure of two tumor necrosis factor inhibitors and in Romania JAKi were positioned at the same level as bDMARDs. Phosphodiesterase-4 inhibitors were not in use or not reimbursed in most countries. Analgesics were not specifically mentioned in several of the national treatment recommendations.
Conclusion: Only a few European countries incorporated all EULAR and ASAS/EULAR treatment recommendations in their national recommendations. The potential impact of this on access to b/tsDMARD treatments needs to be further explored.
To cite this abstract in AMA style:
Michelsen B, Østergaard M, Nissen M, Ciurea A, Moeller B, Ørnbjerg L, Zavada J, Glintborg B, MacDonald A, Laas K, Nordstrom D, Gudbjornsson B, Iannone F, Hellamand P, Kvien T, Rodrigues A, Codreanu C, Rotar Z, Castrejon I, Karlsson Wallman J, Vencovsky J, Loft A, Heddle M, Vorobjov S, Hokkanen A, Grondal G, Sebastiani M, van de Sande M, Kristianslund E, Santos M, Mogosan C, Tomsic M, Diaz-Gonzalez J, Di Giuseppe D, Hetland M. Differences and Similarities Between the EULAR/ASAS-EULAR Recommendations and National Recommendations for Treatment of Patients with Psoriatic Arthritis and Axial Spondyloarthritis Across Europe [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/differences-and-similarities-between-the-eular-asas-eular-recommendations-and-national-recommendations-for-treatment-of-patients-with-psoriatic-arthritis-and-axial-spondyloarthritis-across-europe/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/differences-and-similarities-between-the-eular-asas-eular-recommendations-and-national-recommendations-for-treatment-of-patients-with-psoriatic-arthritis-and-axial-spondyloarthritis-across-europe/