Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II: Diagnosis and Prognosis
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Recently, antibodies directed against carbamylated antigens (anti-CarP antibodies) were identified in rheumatoid arthritis (RA). Studies have established the predictive and prognostic value of this antibody system. As most of the previous reports described the performance characteristics of anti-CarP assay in European populations using a two-step research assay, we analyzed the performances characteristics of a single step anti-CarP assay in US based population of RA subjects and control groups.
Methods: Anti-CarP antibodies were measured using fetal calf serum based single step assay (research use only, Inova Diagnostics, San Diego, USA). The analytical and diagnostic performances of the anti-CarP assay were established using a biobank of specimens collected from consented subjects (640 RA subjects fulfilling the 1987 or 2010 criteria, 197 normal healthy volunteers and 636 other disease subjects). The median age of RA subjects at diagnosis was 60 years and it ranged from 41 to 55 years in control groups. Mean disease duration in RA was 12 years, versus 3 to 12 years in control groups. Anti-citrullinated protein antibodies (ACPA) and rheumatoid factor IgM (RF-IgM) status were tested using EliA CCP and RF IgM assays, respectively (ThermoFisher, Upsala, Sweden). Diagnostic performances in distinguishing RA from other control groups were analyzed using sensitivity, specificity, likelihood ratio (LR) and diagnostic odds ratio (DOR).
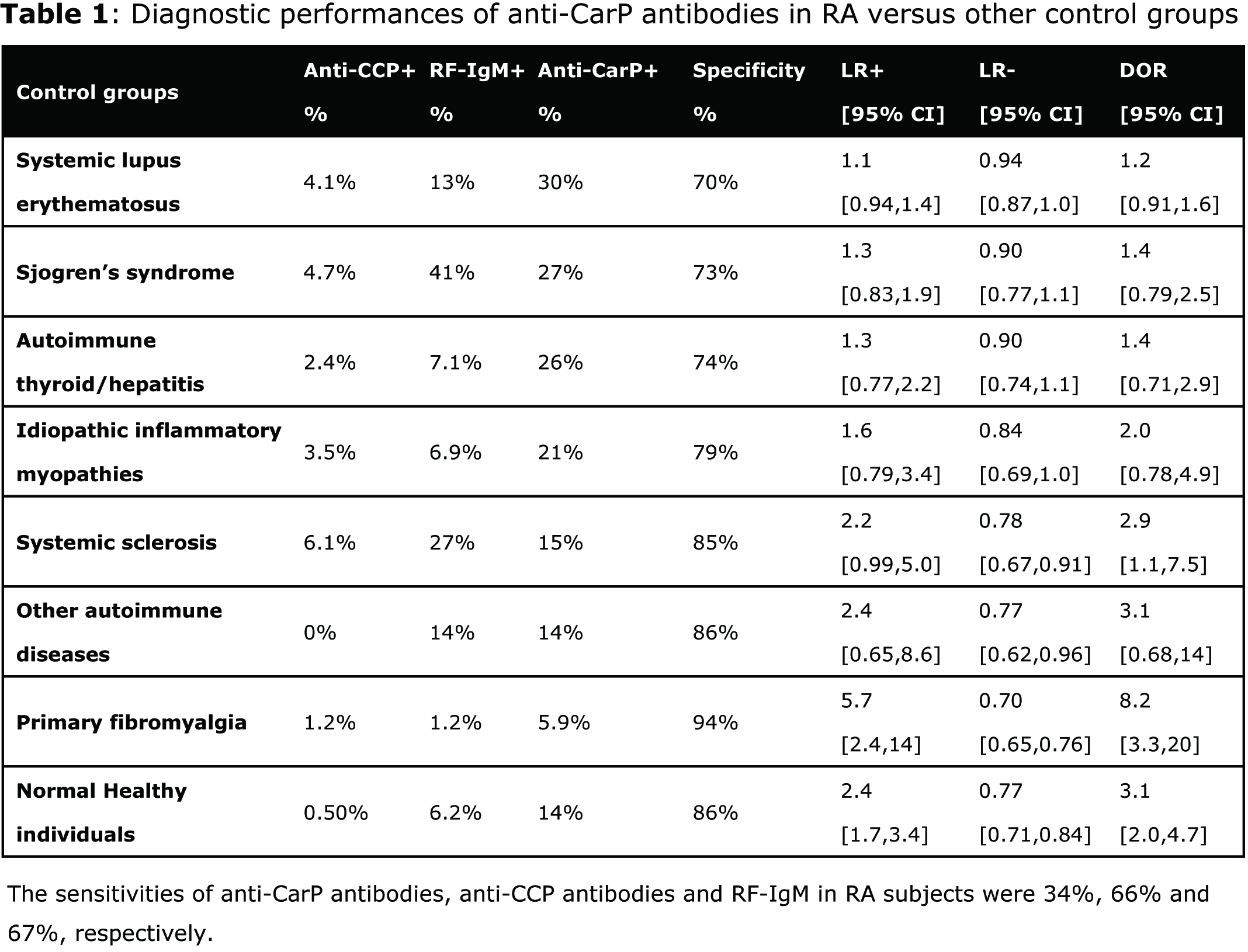
Results: The intra-assay and inter-assay variations of the anti-CarP ELISA were 7.0% and 11%, respectively. Anti-CarP antibodies, anti-CCP antibodies and RF-IgM were present in 34%, 66% and 67% of RA subjects, respectively. The overall specificity of anti-CarP antibodies was 78%. When stratified by anti-CCP status, the prevalence of anti-CarP antibodies was 42% and 16% among anti-CCP positive and negative RA subjects, respectively, a finding consistent with previous studies. The prevalence of anti-CarP antibodies in various control groups ranged from 5.9% to 30% (Table 1), thereby yielding specificities ranging from 70% (systemic lupus erythematosus, SLE) to 94% (primary fibromyalgia). Diagnostic odds ratios (DOR) for RA versus the different control groups ranged from 1.2 to 8.2.
Conclusion: These data replicate previous studies and support the notion that anti-CarP antibodies are helpful in the setting of ACPA negative RA subjects. However, clinicians should be aware of the low specificity of this marker in certain autoimmune rheumatic diseases, particularly SLE.
Disclosure: J. Shi, Exagen Diagnostics, 3; J. Milo, Inova Diagnostics, 3; K. Bradey, Exagen Diagnostics, 3; C. Bentow, Inova Diagnostics, 3; J. Conklin, Exagen Diagnostics Inc., 3; T. O'Malley, Exagen Diagnostics, 3; A. Sace, None; R. Lafon, None; D. Poling, Exagen Diagnostics, Inc., 3; C. Ibarra, Exagen Diagnostics, 1, 3; M. Mahler, Inova Diagnostics, 3; T. Dervieux, exagen, 3.
To cite this abstract in AMA style:
Shi J, Milo J, Bradey K, Bentow C, Conklin J, O'Malley T, Sace A, Lafon R, Poling D, Ibarra C, Mahler M, Dervieux T. Diagnostic Performances of Antibodies Against Carbamylated Proteins in US Based Populations with Rheumatoid Arthritis and Other Autoimmune Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/diagnostic-performances-of-antibodies-against-carbamylated-proteins-in-us-based-populations-with-rheumatoid-arthritis-and-other-autoimmune-rheumatic-diseases/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/diagnostic-performances-of-antibodies-against-carbamylated-proteins-in-us-based-populations-with-rheumatoid-arthritis-and-other-autoimmune-rheumatic-diseases/