Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Patients with PsO and PsA have a higher prevalence of cardiometabolic comorbidities like diabetes compared to the general population. Clinical data have shown apremilast may reduce weight and HbA1c. We describe changes in cardiometabolic parameters and disease activity outcomes over a 6-month follow-up period among PsO and PsA patients stratified by diabetes status and newly treated with apremilast using a large, United States-based real-world cohort.
Methods: We estimated the burden of diabetes among PsO and PsA patients in the OM1 Real-World Data Cloud newly initiating apremilast and persistent for 6 months. These data spanned from March 2013 to November 2021. We stratified the study cohort by diabetes status (no diabetes vs pre-diabetes/type 2 diabetes mellitus [T2DM]). Pre-diabetes was identified by either 2 diagnosis codes at least 30 days apart, HbA1c levels between 5.7% to 6.4%, or fasting glucose levels between 100-125 mg/dL. T2DM was defined as having two diagnosis codes at least 30 days apart or evidence of antidiabetic medication during baseline.
We described demographic and clinical characteristics, comorbidities, and treatments at baseline, assessed changes in weight and HbA1c, and disease activity measures (RAPID3 and Physician Global Assessment [PGA]) at 6 months. We used Wilcoxon Rank sum tests and Chi-square tests for comparisons by diabetes status. Mean proportional changes in cardiometabolic measures and disease severity at 6 months were estimated. Multivariable linear regression was used to assess cardiometabolic measures, adjusting for age, sex, use of anti-hypertensives, lipid-lowering therapies, steroids, and corresponding baseline outcomes.
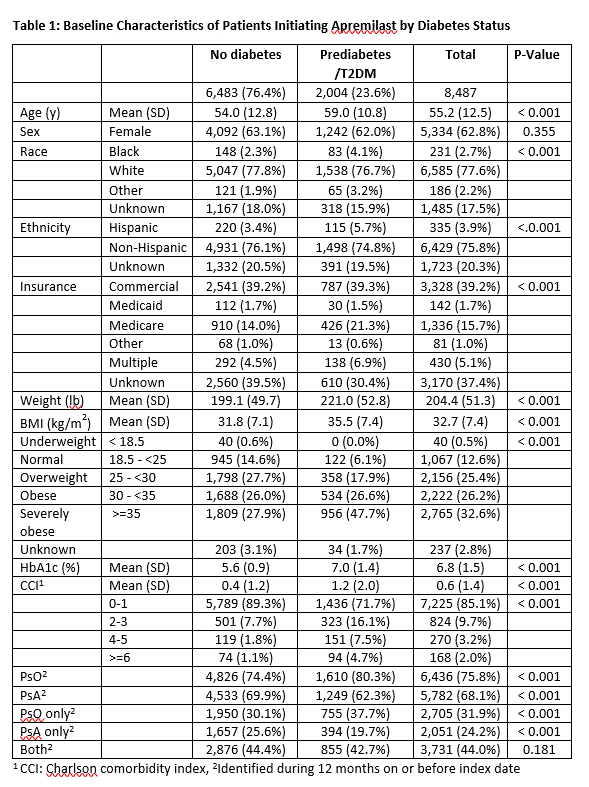
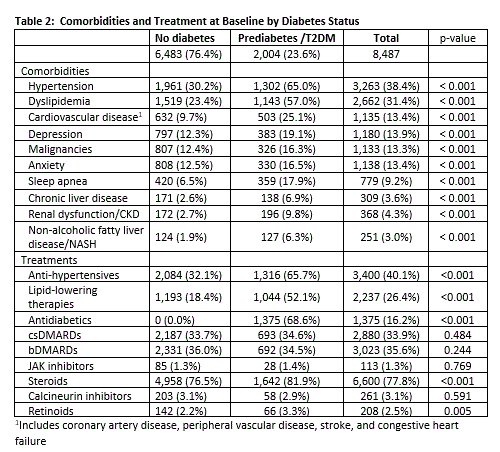
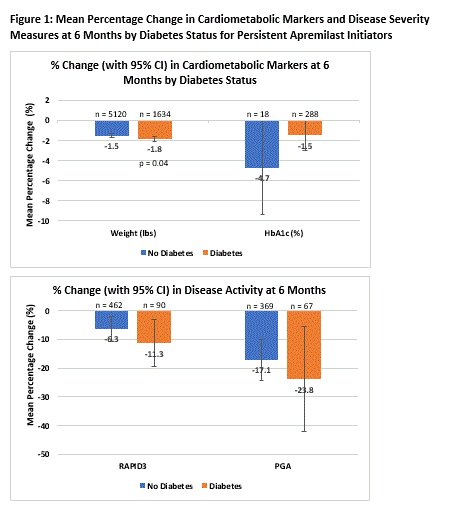
Results: There were 8,487 PsO and PsA patients with outcome measures that initiated apremilast. The mean age was 55.2 years and 62.8% were female. 23.6% (2,004) had pre-diabetes /T2DM. 87.1% (1,746) had T2DM and 12.9% had pre-diabetes. These patients were older (59 vs. 54, p < 0.001), with higher proportions of patients identifying as Black (4.1% vs. 2.3%, p < 0.001) and Hispanic (5.7% vs. 3.6%, p < 0.001) compared to those without diabetes. Most patients were obese (26.2%) or severely obese (32.6%) (Table 1). Comorbidities were prevalent in the diabetes group, with 28.3% having two or more. Prevalent comorbidities included hypertension (38.4%), dyslipidemia (31.4%), and cardiovascular disease (13.4%) (Table 2). Patient and physician outcomes at baseline reflected mild to moderate disease severity. Mean raw RAPID3 score was higher among those with diabetes (13.49 vs. 11.20, p = 0.047), while mean PGA (2.81 vs. 2.77) was similar across strata. We observed reductions in weight, HbA1c and disease severity outcomes at 6 months regardless of diabetes status (Figure 1).
Conclusion: This real-world study highlights the significant cardiometabolic burden among PsO and PsA patients who initiated apremilast. Similar to the clinical trials data, this study suggests apremilast may have beneficial effects on cardiometabolic markers in clinical practice including reductions in weight, HbA1c, and improvements in PsO/ PsA disease outcomes.
To cite this abstract in AMA style:
Orroth K, Cavanaugh C, Qian X, Kumparatana P, Klyachkin Y, Colgan S, Cordey M. Diabetes Burden and Effects of Apremilast on Changes in Cardiometabolic Parameters in Patients with Psoriasis (PsO) or Psoriatic Arthritis (PsA) in a Real-World Setting [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/diabetes-burden-and-effects-of-apremilast-on-changes-in-cardiometabolic-parameters-in-patients-with-psoriasis-pso-or-psoriatic-arthritis-psa-in-a-real-world-setting/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/diabetes-burden-and-effects-of-apremilast-on-changes-in-cardiometabolic-parameters-in-patients-with-psoriasis-pso-or-psoriatic-arthritis-psa-in-a-real-world-setting/