Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Risk stratification in the ACR glucocorticoid-induced osteoporosis guidelines includes DEXA testing, which is not universally implemented at our rheumatology clinic. DEXA utilization should be increased to screen for this effect of long-term glucocorticoid use.
Methods: As a quality improvement project, institutional policy exempted this study from IRB review. As a baseline, charts were reviewed from 50 patients on steroids (equivalent to prednisone ≥5 mg/day, ≥ 3 months) seen in rheumatology clinic in August 2013. DEXA status was categorized as never, current, or out-of-date (>2 years ago). Patient gender, menopausal status, steroid dose, and duration of steroid use were also recorded. Then a medication monitoring questionnaire (see Figure 1) was administered to all patients at the time of visit during the intervention pilot period in January 2014 with the intention of triggering more DEXA orders by providers. Other drugs and monitoring tests were included on the form for clinical utility but were not measured for this project. Charts from the 44 patients on qualifying steroid therapy seen during the pilot period were reviewed.
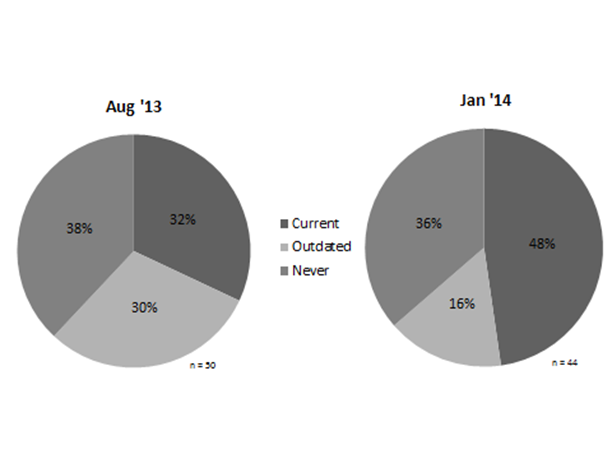
Results: During the baseline period, 32% of patients had a current DEXA. Status distribution was similar regardless of menopausal status or prednisone dose (>5 versus ≤5 mg/day). Of 9 male patients, 7 (78%) had current DEXA as opposed to 10 of 41 female patients (24%). During the intervention period, current DEXAs (including DEXAs ordered at the visit) increased to 48% (see Figure 2). The number of patients with “never” status was similar; most of the gain to “current” status resulted from updating DEXA testing.
Conclusion: A medication monitoring questionnaire at routine clinic visits can serve as a reminder to trigger appropriate DEXA orders in patients on long-term steroids. This intervention could be modified for other chronic medication monitoring parameters as well. Future interventions will target increasing FRAX calculation and documentation.
Figure 1. Medication monitoring questionnaire
Figure 2. DEXA status during baseline and pilot periods
Disclosure:
B. Scholz,
None;
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dexa-testing-in-long-term-steroid-use/