Session Information
Date: Monday, November 14, 2022
Title: Abstracts: Vasculitis – Non-ANCA-Associated and Related Disorders II
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Disease activity indices that accurately assess inflammation in Takayasu’s arteritis (TAK) are needed. While clinical assessment is often considered the gold standard, determining if a symptom represents active vasculitis or damage can be challenging in TAK. FDG-PET can directly assess vascular inflammation but specificity of imaging findings, especially in absence of corresponding clinical symptoms, is questionable. We present the development of the Takayasu’s Arteritis Integrated Disease Activity Index (TAIDAI).
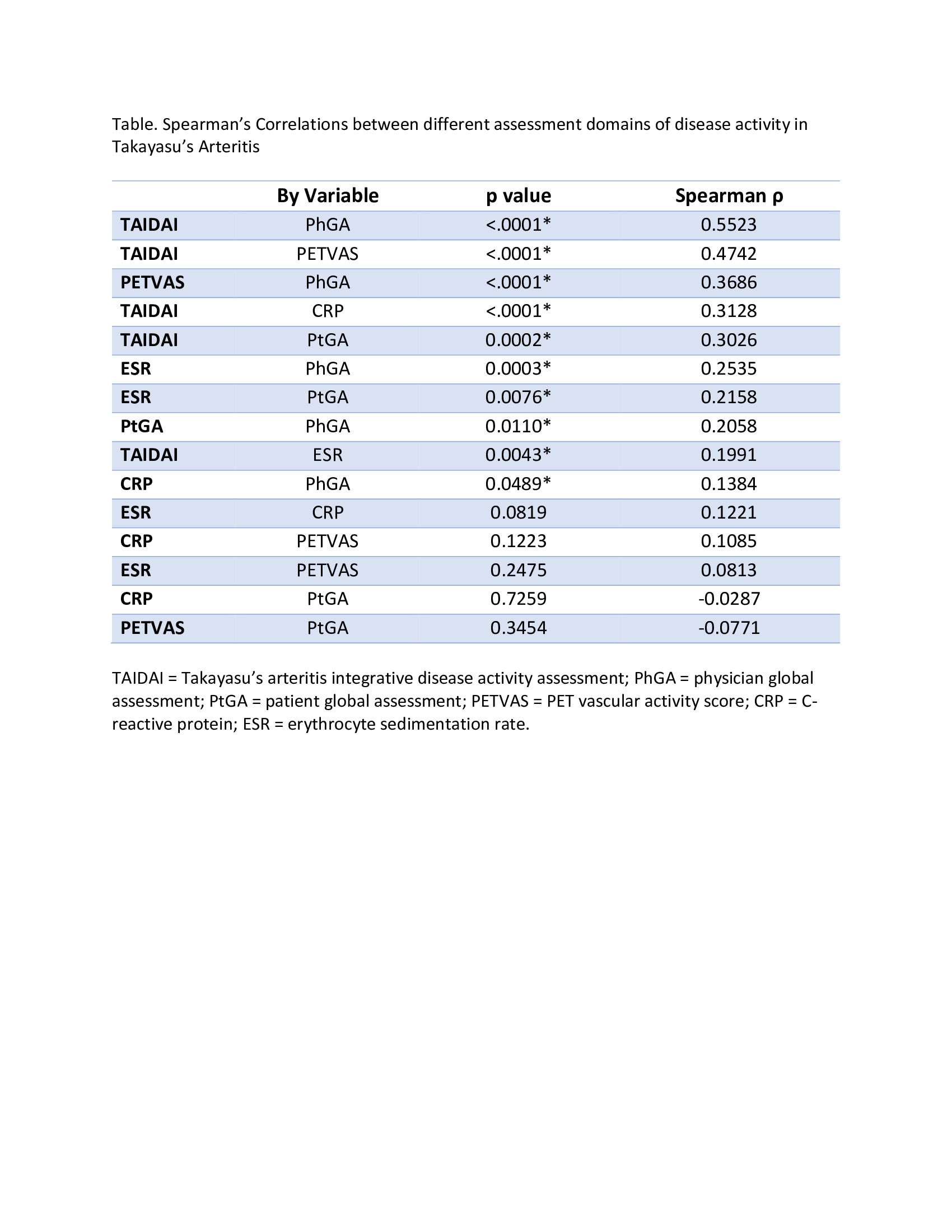
Methods: A comprehensive literature review informed selection of TAK-associated clinical symptoms for TAIDAI. Symptoms present within 7 days of clinical evaluation were recorded in an observational cohort study in TAK. Two clinicians independently recorded presence of each symptom without determining whether the symptom reflected active disease or damage. Physician global assessment (PhGA) of clinical disease activity (active vs remission on scale of 0-10) and certainty of assessment (certain vs uncertain) were recorded. Each patient underwent FDG-PET at every visit. Active vasculitis, defined as arterial FDG uptake > liver, was recorded in specific arterial territories and quantified using the PET Vascular Activity Score (PETVAS). To score TAIDAI, clinical symptoms are paired with specific arterial territories and only scored if there is active vasculitis by FDG-PET in a corresponding arterial territory (Figure). Constitutional symptoms (fever, fatigue, weight loss) are scored only if CRP ≥10mg/L or ESR≥40mm/hour. To determine content validity, correlations were performed between PhGA, patient-reported global assessment (PtGA, 0-10 scale), ESR, CRP, PETVAS, and TAIDAI. To determine criterion validity, performance characteristics of TAIDAI were calculated relative to clinical assessment, restricted to cases of assessment certainty. To determine responsiveness, TAIDAI scores were assessed longitudinally in patients initiated on tumor necrosis factor inhibitors (TNFi) or tocilizumab (TCZ). Complete response was defined as resolution of all symptoms of active vasculitis plus a daily prednisone dose not exceeding >50% of the pre-treatment visit.
Results: Eighteen clinical symptoms were identified for inclusion in the index. TAIDAI was calculated for 96 patients over 204 visits. Clinically active disease was present in 62 (30%) visits, with uncertainty of assessment in 82 (40%) visits. Inter-rater agreement for PhGA was poor (45%). At baseline, median TAIDAI was 1 (range 0-9), and 48 (50%) patients had a TAIDAI of 0. TAIDAI score correlated most strongly with all other assessments (Table). TAIDAI > 0 defined active disease with 96% sensitivity and 77% specificity compared to clinical assessment. Complete response was observed in 14/17 patients treated with TNFi and 6/9 patients treated with TCZ. A TAIDAI score of 0 was achieved by all responders, and TAIDAI remained > 0 for 10/12 treatment failures.
Conclusion: TAIDAI is a novel disease activity index focused on scoring clinical symptoms only when supported by relevant imaging or laboratory data. TAIDAI should be tested in other cohorts and future clinical trials in TAK.
To cite this abstract in AMA style:
Marvisi C, Bolek E, Ahlman M, Alessi H, Redmond C, Merkel P, Salvarani C, Quinn K, Grayson P. Development of the Takayasu’s Arteritis Integrated Disease Activity Index [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/development-of-the-takayasus-arteritis-integrated-disease-activity-index/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-the-takayasus-arteritis-integrated-disease-activity-index/