Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: An international multi-disciplinary effort is underway to develop rigorous, new, consensus- and evidence-based classification criteria for Antiphospholipid Syndrome (APS). The methodological approach includes four phases; we have previously presented Phase I (item generation) and II (item reduction), resulting in 27 candidate criteria organized into laboratory and clinical domains. Phase III (item weighting/threshold identification) is currently underway; here we report initial Phase III case collection results.
Methods: We used REDCap, a secure web-based data system, for Phase III international case collection. The 27 candidate criteria items from Phase II were represented in a standardized case collection form. We asked 17 physicians specializing in APS from Europe, North and South America to provide cases and to rate them using a Likert scale from +3 to -3 (highly likely to highly unlikely to be APS). Cases with higher scores (+2 or +3) were categorized as “highly likely” APS based on treating physician assessment, whereas lower scores (+1 to -3) were categorized as “equivocal or unlikely” APS. We calculated risk ratios to represent the probability that a laboratory or clinical candidate criterion would be associated with a “highly likely” compared to “equivocal or unlikely” APS case.
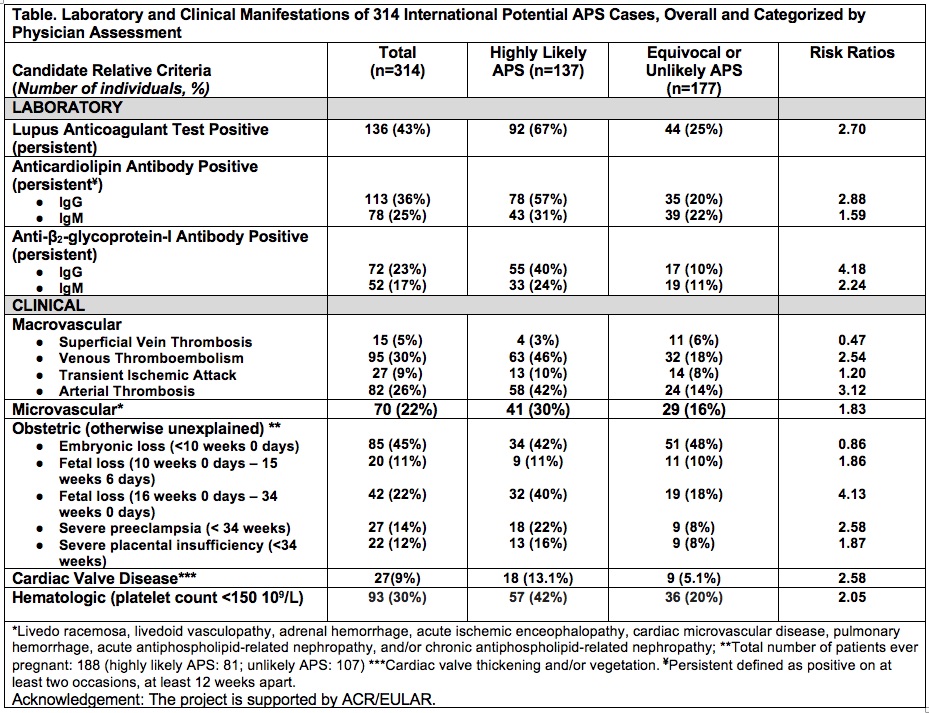
Results: We collected 314 potential APS cases (mean age 43.8 +/- 14.4 years; 79% female; and 77% white) between 6/2019-8/2019 from 17 sites in Europe (n=8, 47%), North America (n=7, 41%), and South America (n=2, 11%). Majority of cases were potential primary APS (n=201, 64%). Out of 314 cases, 137 (44%) were rated as “highly likely” and 177(56%) as “equivocal or unlikely” APS. The table demonstrates frequency of laboratory and clinical manifestations of potential APS cases (overall and categorized by physician assessment) as well as the risk ratios; the three criteria with the lowest risk ratios (< 1.5) were superficial vein thrombosis, transient ischemic attack, and embryonic loss < 10 weeks 0 days.
Conclusion: In Phase III of the development of new APS classification criteria, a large international collection of cases spanning the spectrum of “highly likely” to “equivocal or unlikely” APS was used to identify the observation frequency of each of the candidate criterion from Phase II in real world clinical settings. In next steps, these proposed candidate criteria will be further refined and weighted using multi-criteria decision analysis methodology, and a preliminary threshold for APS classification will be determined.
To cite this abstract in AMA style:
Barbhaiya M, Zuily S, Ahmadzadeh Y, Costenbader K, Naden R, Erkan D. Development of New International Classification Criteria for Antiphospholipid Syndrome: Phase III Case Collection Results [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/development-of-new-international-classification-criteria-for-antiphospholipid-syndrome-phase-iii-case-collection-results/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-new-international-classification-criteria-for-antiphospholipid-syndrome-phase-iii-case-collection-results/