Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
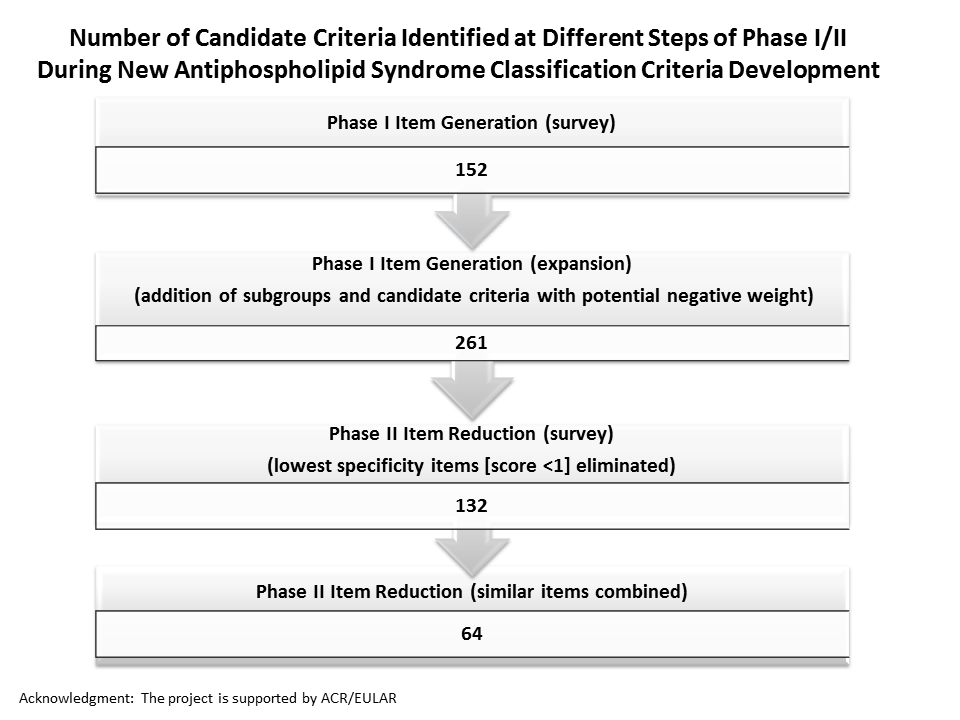
Background/Purpose: An international multidisciplinary effort has been initiated to develop new rigorous, consensus-based criteria to identify patients with high likelihood of having APS. The methodological approach includes both expert-based and data-driven methods, and four phases: item generation (I); item reduction (II); item weighting and threshold identification (III); and classification criteria refinement and validation (IV). We have previously reported Phase I results (Barbhaiya M, et al. Lupus 2016;25[Supp1S]:11); here we report our Phase II “Item Reduction” survey results. The aim of Phase II is to reduce the 261 Phase I items to approximately 20 items, considered to be the most important features distinguishing APS from other conditions.
Methods: 61 international physician-scientists experienced in APS were e-mailed a survey to rank each item generated in Phase I on a Likert scale (-5 to +5), with (-5) extremely strongly against APS, i.e., more likely related to another disease entity; (0) not for or against APS; and (+5) extremely likely APS. Experts were asked to “consider two patients who are exactly the same except that one has the clinical feature presented and the other does not. Please rate each feature in terms of how strong this feature is in differentiating APS from other similar conditions, i.e., specific for APS.” An article reference library (n: 26), including mostly aPL/APS-related systemic reviews and meta-analyses, was provided. Mean survey scores for each item (±SD) were calculated. Items were ranked from highest to lowest mean score, and highest scoring items were grouped based on independent logical domains.
Results: Phase II survey response rate was 71%. Of 43 responders, 22 were rheumatologists, four hematologists, four nephrologists/cardiologists/neurologists/vascular specialists, four internists, three clinical immunologists, three pediatric rheumatologists, two pediatric hematologists, and one obstetrician. Mean item scores ranged from -2.29 to 4.65; items of low specificity (score less than 1) were eliminated. The higher specificity items (n=132) were organized into seven clinical (cardiopulmonary, dermatologic, hematologic, neurologic, obstetric, renal/abdominal, and vascular), two laboratory (antiphospholipid antibody [aPL] ELISA and lupus anticoagulant), and one pathologic domains. Items overlapping or describing similar concepts were combined (n=64) (Figure).
Conclusion: Sixty-four candidate criteria identified using the Phase II item reduction survey will be further reduced and refined based on the ongoing project to develop new APS classification criteria, with the goal of better standardizing patients for APS research.
To cite this abstract in AMA style:
Barbhaiya M, Zuily S, Ahmadzadeh Y, Naden RP, Costenbader K, Erkan D. Development of New International Classification Criteria for Antiphospholipid Syndrome: Phase II Item Reduction Survey [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/development-of-new-international-classification-criteria-for-antiphospholipid-syndrome-phase-ii-item-reduction-survey/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-new-international-classification-criteria-for-antiphospholipid-syndrome-phase-ii-item-reduction-survey/