Session Information
Date: Sunday, November 8, 2015
Title: Vasculitis Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Patients with
anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides
(AAVs), including granulomatosis with polyangiitis (Wegener’s, (GPA), eosinophilic
granulomatosis with polyangiitis
(Churg-Strauss, EGPA), and microscopic polyangiitis (MPA), can face persistent disease activity, disease-associated
damage, or adverse treatment effects, all of which may impact on health related
quality of life. There is currently no disease-specific patient-reported
outcome (PRO) for AAV. The development of a PRO should be in compliance with FDA
recommendations and involves: (i) Questionnaire item development; (ii) Item
reduction and scale generation; and (iii) Testing scale properties –
reliability, validity and responsiveness. Following these principles, a
multi-national collaboration of researchers and patient-partners from the UK,
USA and Canada has been conducting the first stage of questionnaire item development with the
aim of creating a tool with content validity (and cultural/linguistic
equivalence) appropriate for use in all three countries.
Methods: Exploratory
semi-structured patient interviews were performed in the UK, USA, and Canada.
The aim was to identify salient dimensions of quality of life and perceived
problems of health status related to having AAV. The overall sample size was
determined by the point at which no new themes emerged from interviews
(saturation), and was also guided by a purposive sampling framework to ensure a
range of participants were included (for
example differing disease presentations, age and genders). Researchers (within
and across research groups) independently scrutinised
interview transcripts for relevant themes. Themes identified from transcripts
were then re-cast as candidate questionnaire items. Regular teleconferences
maintained equivalence of methods and exchange of relevant themes.
Results: Forty-nine
semi-structured interviews of patients with AAV were conducted. After
transcription and scrutiny, 60 distinct themes related to having AAV were
identified, these included symptoms (related to condition or treatment) and the
ways in which these symptoms influenced patients’ ability to work, activities
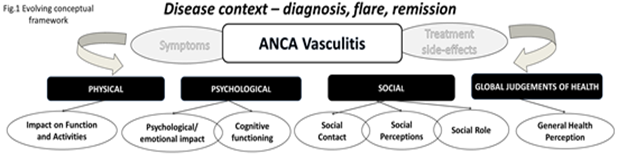
of daily living, engagement in social activities, and state of mind. The
interaction between these factors is demonstrated in the evolving conceptual
framework for the PRO, shown in Figure 1.
Conclusion: A list of
themes and candidate items, drawn directly from patient experience, has been
used to inform the development of a PRO for AAV. A parallel survey of~500
patients in the UK and US is currently underway and will produce an instrument
with appropriate scale structure, measurement properties, and scoring
algorithms. This will be followed by a multi-centred
prospective validation study.
To cite this abstract in AMA style:
Robson J, Ashdown S, Dawson J, Easley E, Gebhart D, Kellom K, Lanier G, McAlear C, Milman N, Peck J, Shea JA, Tomasson G, Luqmani R, Cronholm PF, Merkel PA. Development of an Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis Patient-Reported Outcome Measure: Identification of Salient Themes and Candidate Questionnaire Item Development [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/development-of-an-anti-neutrophil-cytoplasmic-antibody-associated-vasculitis-patient-reported-outcome-measure-identification-of-salient-themes-and-candidate-questionnaire-item-development/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-an-anti-neutrophil-cytoplasmic-antibody-associated-vasculitis-patient-reported-outcome-measure-identification-of-salient-themes-and-candidate-questionnaire-item-development/