Session Information
Date: Monday, November 13, 2023
Title: (1082–1099) Measures & Measurement of Healthcare Quality Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Even though individuals with inflammatory rheumatic diseases (IRDs) were excluded from the SARS-CoV-2 vaccine trails, studies have shown that these individuals at risk of a severe course of infection do show an immune response after vaccination. The goal of our study was to show the development of the adaptive immunity over time and its activity towards different variants of concern (VoC).
Methods: For this retrospective study, patients with IRDs and a healthy control group were recruited. To evaluate the immunity induced by SARS-CoV-2 vaccination the antibody titres against the S1-domain of the spike protein of SARS-CoV-2 (BAU/ml) and the IC50 neutralization of different VoCs (EU1, Delta, Omicron-BA.5, BQ1.1, BF.7) were quantified at different time points before and after each vaccination. The study period started before the first and ended 26 weeks after the third vaccination.
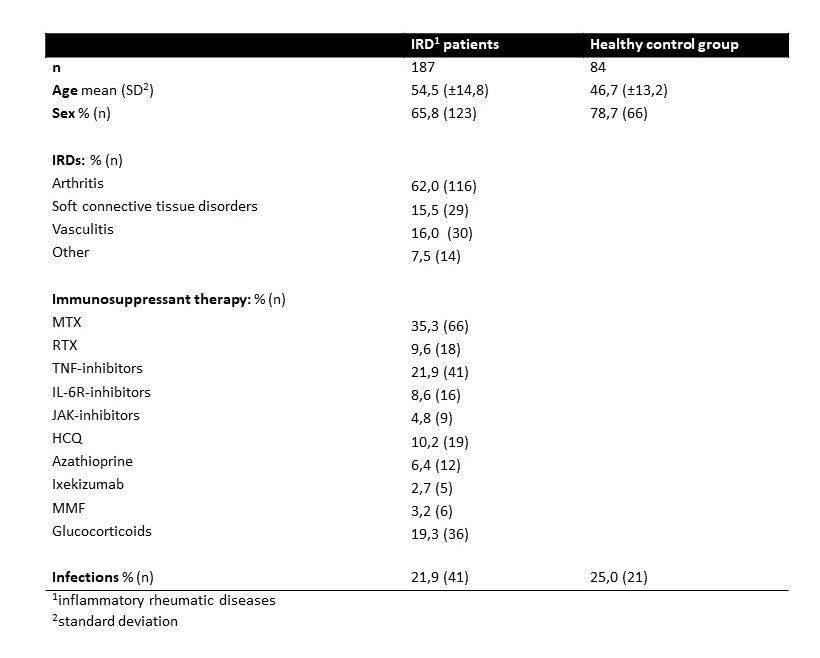
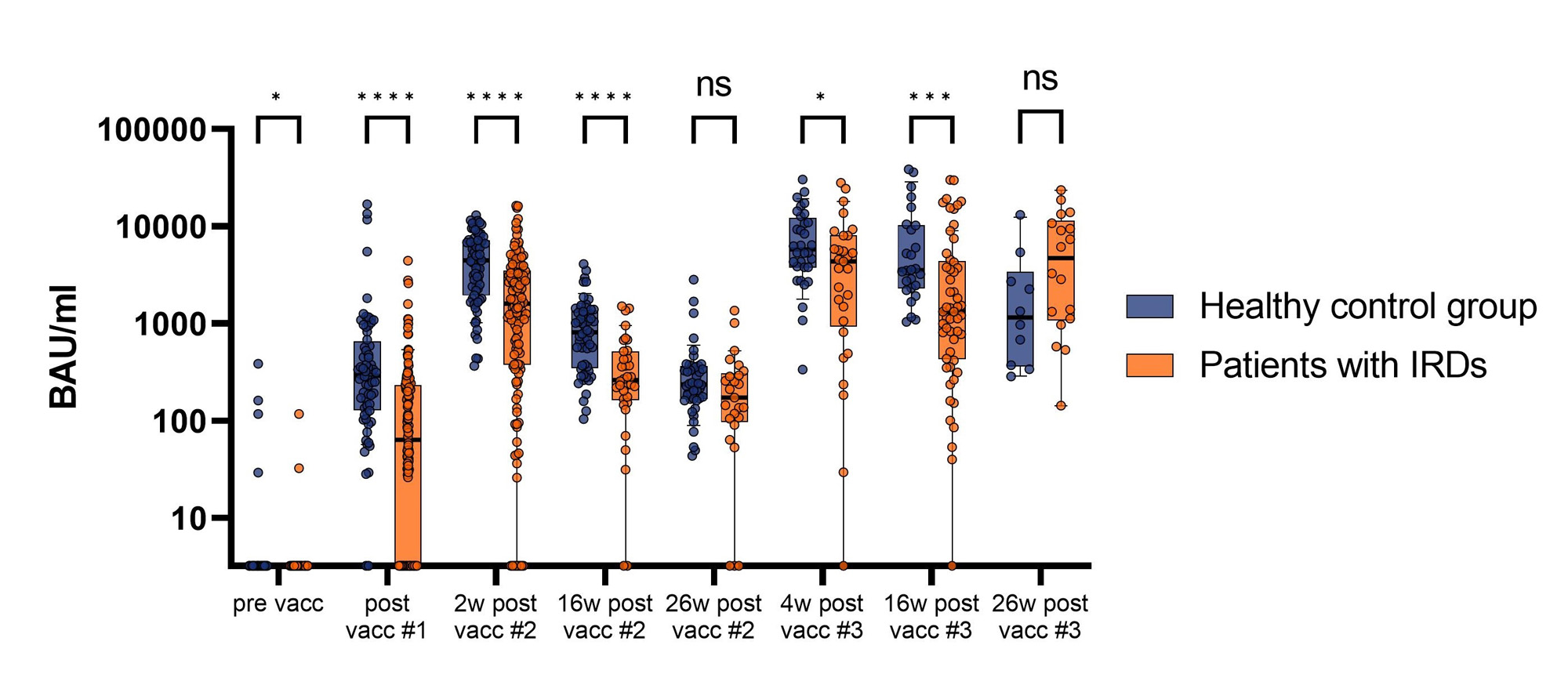
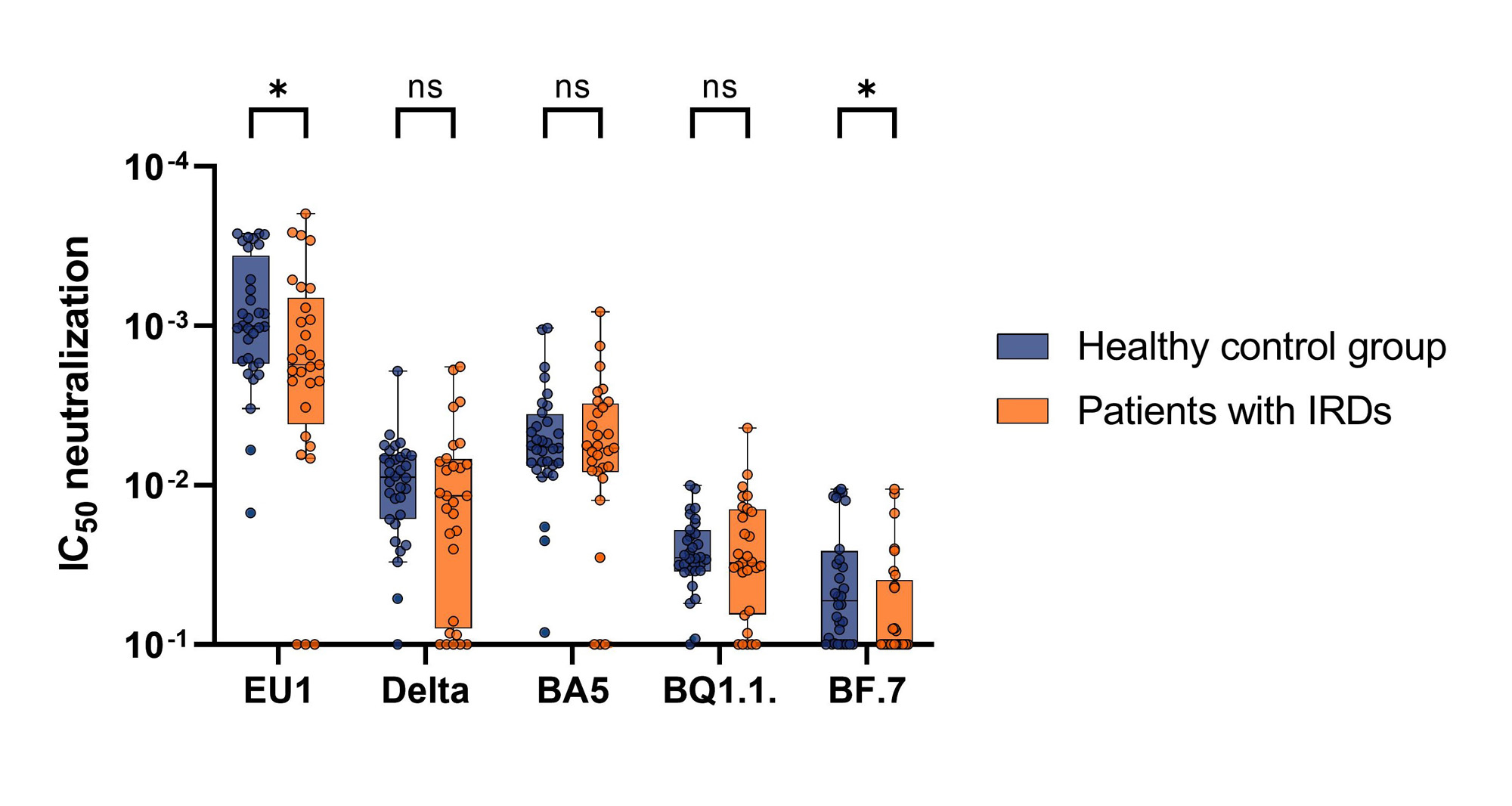
Results: In total 187 patients with IRDs [mean age (standard deviation (SD)) 54.5 (±14.8) years, 65.8% female] and 84 healthy controls [46.7 (±13.2) years, 78.7% female] were included into the study (Table 1). Even though both patients and controls showed high antibody titres after the first two vaccinations [median (interquartile range (IQR)) 1600 BAU/ml (378.2-3468); 4446 BAU/ml (1943-7200)] the titres of the patients were significantly lower (P< 0.0001). After declining over the next 24 weeks, the titres showed another significant rise after the third vaccination in both groups [patients: 4344 BAU/ml (933.2-8023); controls: 5795 BAU/ml (3779-12209)]. However, the levels reached by the patients were still significantly lower compared to the controls (P=0.04). The decline of titres after the third vaccination was not as operand as after the second in either group (Fig. 1). Both groups displayed robust neutralization activities after the first two vaccinations for EU1, Delta and BA.5 that increased after the third dose of the mRNA vaccine. However, the controls showed a rise in neutralization capacity until 16 weeks after the second vaccination while it already started declining after the 2-week measurement in patients resulting in a significant difference. Notably, neutralization of Delta and BA.5 were significantly lower compared to the ancestral EU1. Good neutralization capacity of BQ1.1 in both groups were only reached after the third vaccination, while neither group presented reliable neutralization of the highly immunoevasive BF.7 after any vaccination. (Fig. 2) Individuals treated with rituximab were not able to mount a reliable humoral immune response after any vaccination. However, they did show adaptive T-cell responses which were comparable to the controls.
Conclusion: Although patients with IRDs show lower anti-SARS-CoV-2 spike antibody titres and partly lower IC50 neutralization compared to healthy controls, most of them mount a robust immune response against SARS-CoV-2, indicating some level of protection from a severe course of infection. However, the original vaccines did not induce a robust neutralization capacity for the recently emerged BF.7 variant in patients or controls. Thus, variant-adapted vaccines should be considered, in particular, in high-risk patient groups, including individuals with IRDs.
To cite this abstract in AMA style:
Huppke L, Wratil P, Bischof M, Wolfrum S, Ullrich F, Singh D, Lichtnekert J, Grümme L, Gebhardt C, Krüger K, Wiesent F, Skapenko A, Keppler O, Schulze-Koops H. Development of Adaptive Immunity Against Different SARS-CoV-2 Variants over the Course of Three COVID-19 Vaccinations in Patients with Inflammatory Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/development-of-adaptive-immunity-against-different-sars-cov-2-variants-over-the-course-of-three-covid-19-vaccinations-in-patients-with-inflammatory-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-adaptive-immunity-against-different-sars-cov-2-variants-over-the-course-of-three-covid-19-vaccinations-in-patients-with-inflammatory-rheumatic-diseases/