Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: Patient Outcomes, Preferences, & Attitudes I: Assessment Tools
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Standard of care therapy for SLE relies heavily on broad-spectrum immune suppressants. Therapeutic drug development is critical to the approval of targeted therapies; however, patient recruitment in lupus clinical trials faces many challenges that include patient, provider, and community barriers, as well as clinical trial design constraints. Here we describe the development of a decision aid (DA) for participation in clinical trials, outlining the choice to continue with standard of care (SOC) vs. enroll in the clinical trial for the treatment of moderate to severe SLE disease activity or flares.
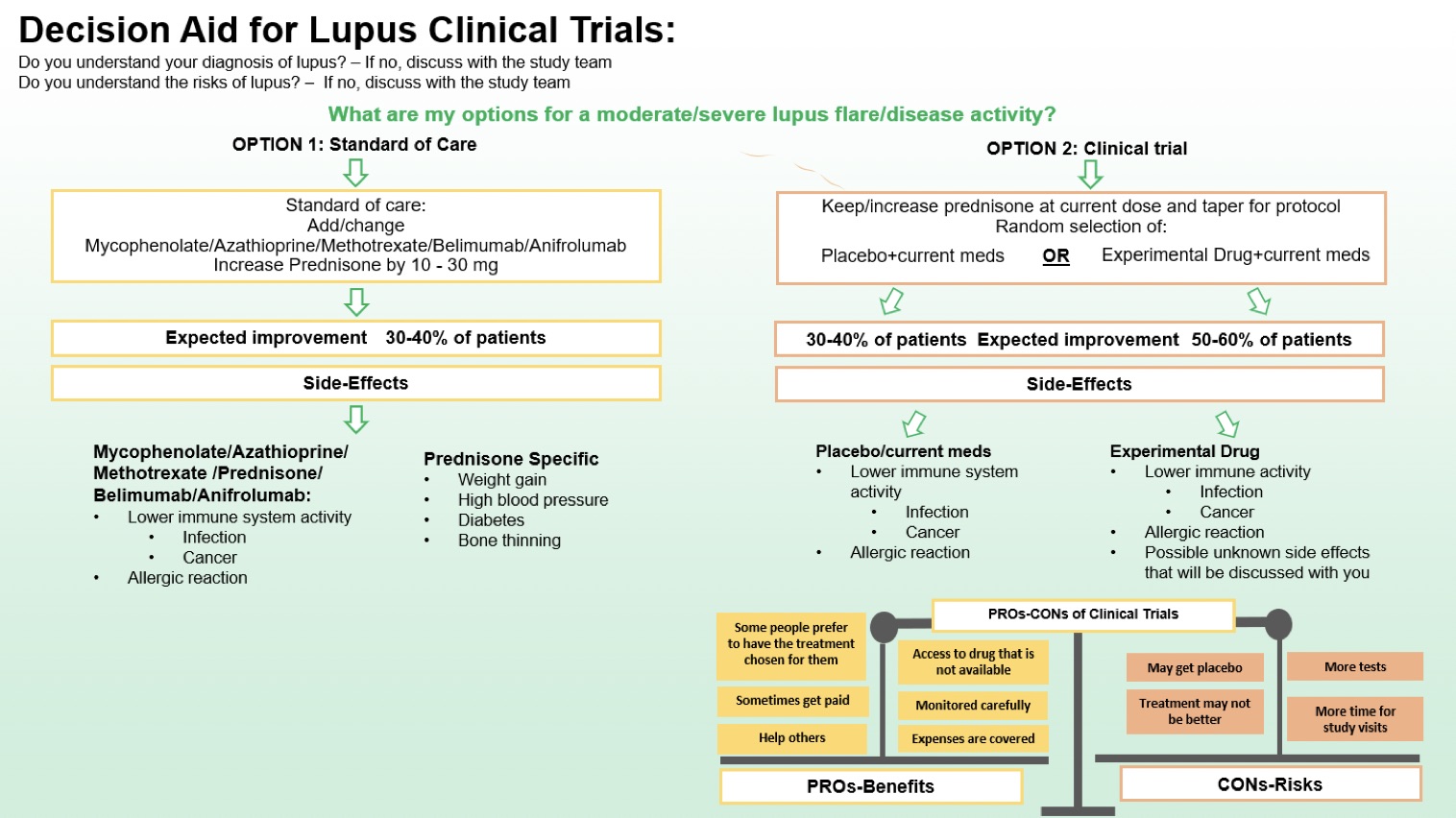
Methods: The DA was developed using a collaborative, iterative process. The development working group included lupus clinical trialists, clinicians, methodologists, and patients. The initial draft based on the International Patient Decision Aid Standards (IPDAS) guidelines was evaluated and refined in semi-structured focused interviews with 10 lupus providers and 10 patients and working version of the decision aid was finalized, see Figure 1. Patients and providers were asked if the components of the DA, including definitions of treatments, expected improvement were clear (yes, no, unsure) and helpful (on a scale of 0-5). Interviews were conducted until thematic saturation was achieved. Responses on usefulness were accumulated, and mean usefulness scores were calculated.
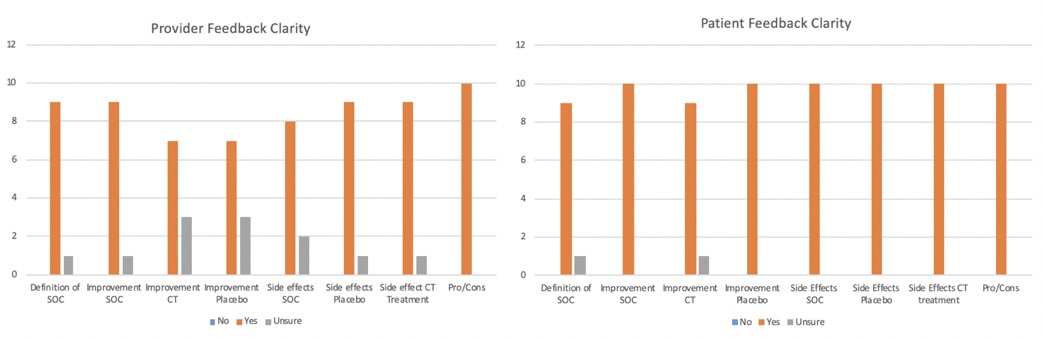
Results: The 10 providers (9 physicians and one advanced practice provider) that participated in the focus group were well versed in the care of patients with lupus and had a mean of 8.1 years of practice. All 10 patients met the 2019 ACR/EULAR criteria for SLE and had been involved in clinical trials, clinical research, and advocacy work in the past. Their mean disease duration was 9 years. Findings of the semi structured interviews are summarized in Figure 2: 90% of providers and patients reported that the definition of SOC treatment was accurate. Additionally, the expected improvement for SOC (90% of providers, 100% of patients), clinical trial drug (70%, 90%), and placebo were clear (70%,100%). Side effects of SOC (80%,100%), placebo (90%, 100%), and clinical trial drug treatment (90%,100%) were also noted to be clear. 100% of providers and patients thought that the figure outlining pros/cons of participating in clinical trials was appropriate. The overall mean usefulness scores were 4.45/5 for providers and 4.72/5 for patients.
Conclusion: We developed a lupus clinical trial DA for patient-provider shared decision-making outlining the choice to continue with SOC vs. enroll in a clinical trial for the treatment of moderate to severe SLE as required by the majority of SLE clinical trials. More data on the usability and performance of the DA is needed to fully understand its role in clinical trial participation.
To cite this abstract in AMA style:
Khalili L, Kukafka R, Geraldino-Pardilla L, Schmidt N, Inzerillo S, Weiner J, Tang W, Askanase A. Development of a Decision Aid for Clinical Trial Participation in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/development-of-a-decision-aid-for-clinical-trial-participation-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-decision-aid-for-clinical-trial-participation-in-systemic-lupus-erythematosus/