Session Information
Date: Sunday, November 10, 2019
Title: 3S107: Pediatric Rheumatology – Clinical I: Systemic JIA (915–920)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: The Juvenile Arthritis Disease Activity Score (JADAS) has gained increasing popularity for the measurement of the level of disease activity in patients with juvenile idiopathic arthritis (JIA). However, so far the JADAS has been validated only in children with the non-systemic categories of JIA. The objective of our study has been to develop and validate the systemic JADAS (sJADAS), a new version of the JADAS specific to systemic JIA (sJIA).
Methods: The sJADAS is made up by adding a fifth item, named Systemic Manifestation Score (SMS), to the four items included in the original tool (physician global assessment of disease activity, parent/patient global assessment of well-being, active joint count and erythrocyte sedimentation rate). The SMS ranges from 0 to 10 and is aimed to quantify the activity of systemic features. The sJADAS score ranges from 0 to 50. The validation sample included patients with definite and possible/probable sJIA with active systemic manifestations, which should comprise fever, who were assessed at baseline and then at a subsequent visit, 2 weeks to 3 months after initial evaluation. Validation procedures included assessment of concurrent, construct and discriminant validity, internal consistency and responsiveness to clinical change.
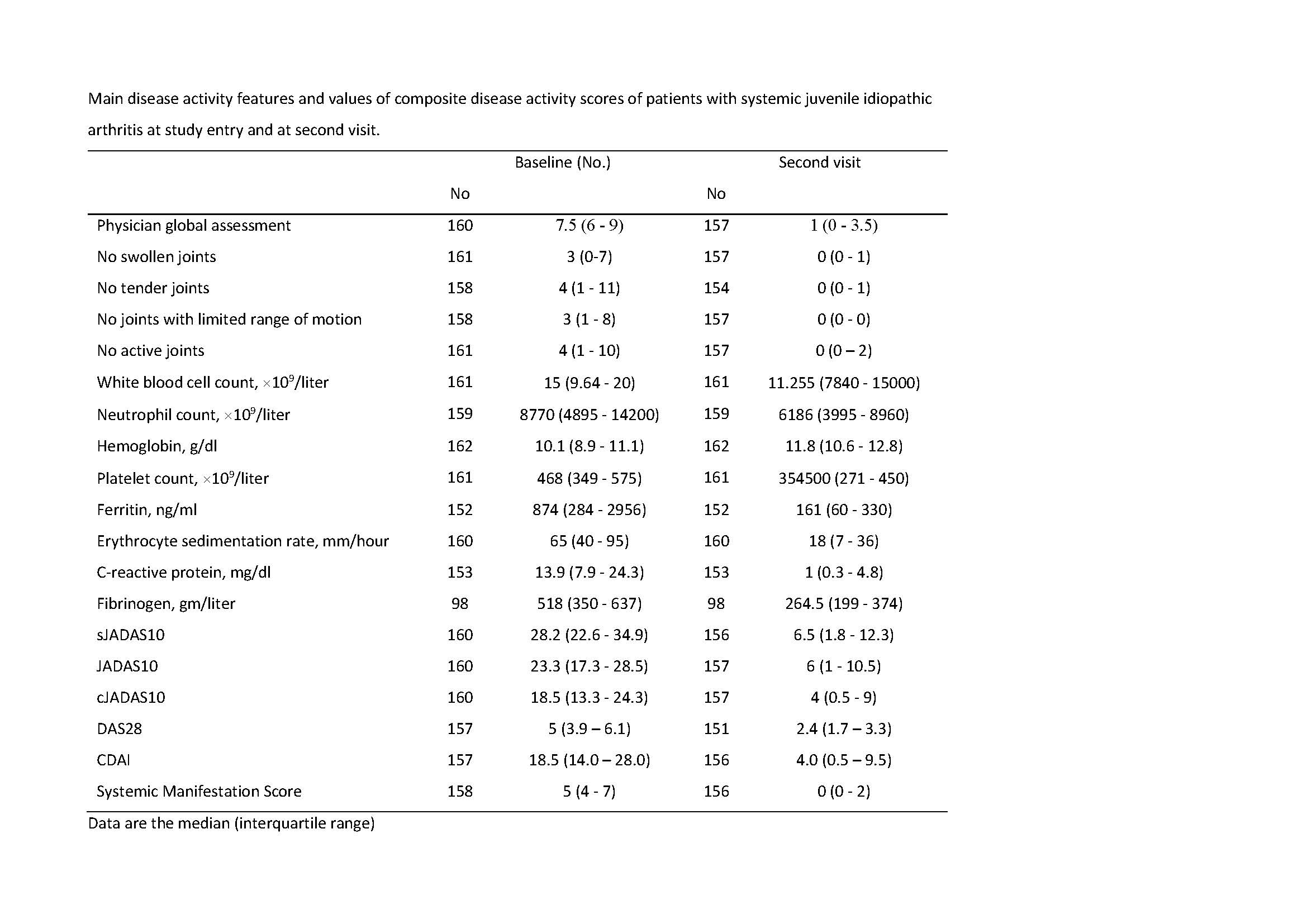
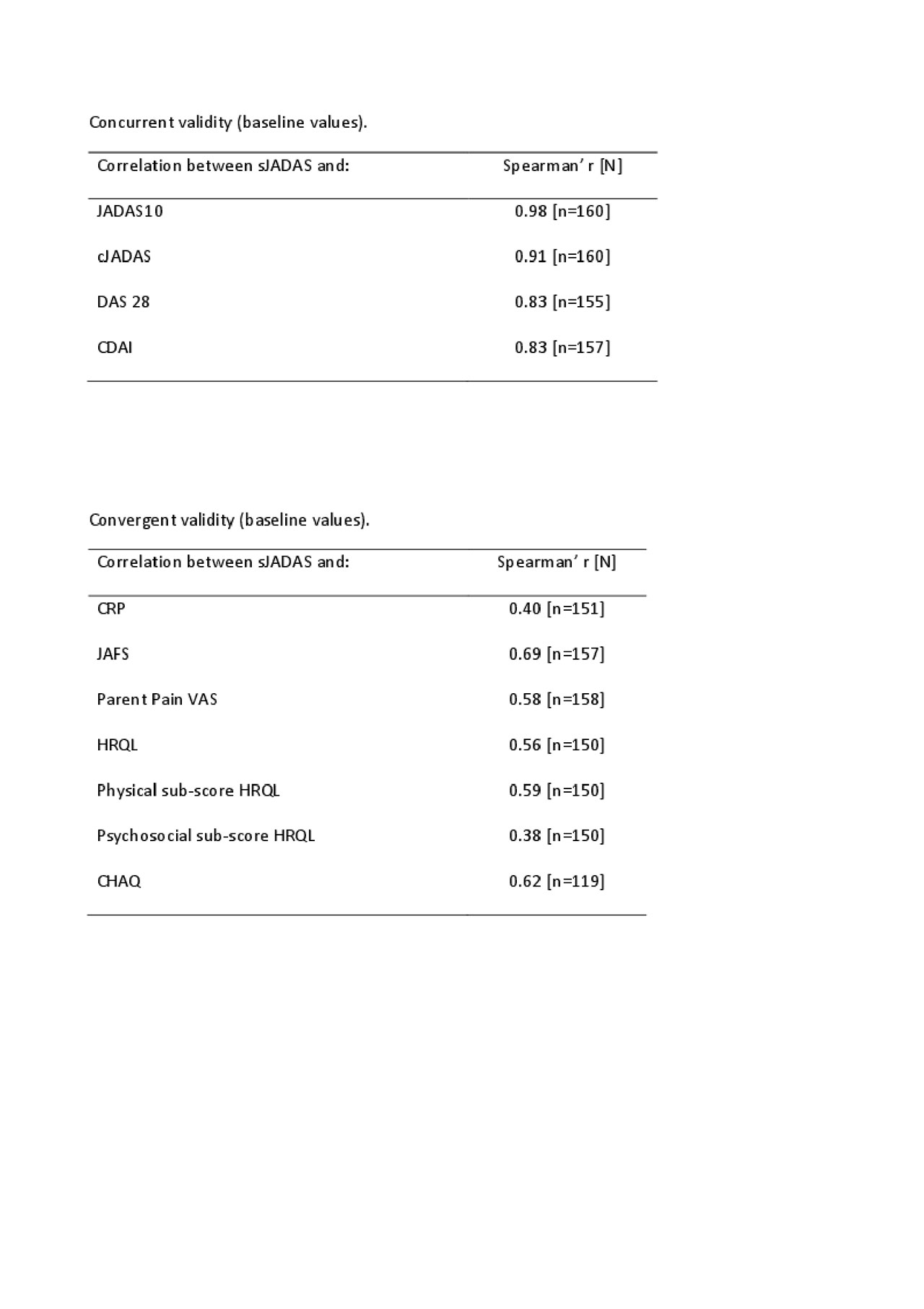
Results: A total of 163 patients, 86.9% (n=139) with definite sJIA and 13.1% (n=21) with possible/probable sJIA, assessed at disease onset (n=91; 55.8%) or at time of a disease flare (n=72; 44.2%) were enrolled in 57 centers in 10 countries from February 2017 to December 2018. Median age at disease onset was 4.9 years (interquartile range, IQR 2.6–7.9) and median age at study entry was 6.4 years (IQR 3.7–10.8). Median disease duration from onset to study entry was 0.2 years (IQR 0.1–1.9). Median sJADAS at baseline visit was 28.2 (IQR 22.8–34.9), while median JADAS10 and clinical JADAS10 (cJADAS10) were respectively 23.3 (IQR 17.3–28.5) and 18.5 (IQR 13.3-24.3). sJADAS correlated strongly with JADAS10 (rs=0.98), cJADAS10 (rs=0.91), DAS28 (rs=0.83) and CDAI (rs=0.83); moderately with functional ability scales (JAFS and CHAQ) (rs=0.69; rs=0.62) and total score (rs=0.56) and physical (rs=0.59) and psychosocial (rs=0.38) subscale scores of the health-related quality of life tool (PRQL) and with pain VAS (rs=0.58); mildly with CRP (rs=0.40). sJADAS discriminated well between patients with or without morning stiffness (p< 0.0001), with different levels of disease activity defined by the physician (p< 0.0001) and with different degrees of pain (p< 0.0001). Internal consistency was good (Cronbach’s alpha=0.635) and comparable to that of JADAS10 (Cronbach’s alpha=0.595). Responsiveness to change, measured on all patients (SRM=2.21) and on patients classified as improved at second visit (SRM=2.59) was strong and superior to that of JADAS10 (SRM=1.97 and 2.28, respectively). Conclusion: The sJADAS was found to be a valid instrument for the assessment of disease activity in sJIA. This score is feasible and easily applicable in standard clinical practice, which should result in its widespread acceptance and use. The good responsiveness to clinical change indicates that the sJADAS is suitable to assess therapeutic response in sJIA clinical trials.
To cite this abstract in AMA style:
Tibaldi J, El Miedany Y, Pal P, Vilaiyuk S, Khubchandani R, Pardeo M, Sabui T, Sawhney S, Russo R, Sztajnbok F, Cimaz R, Minoia F, Alsuweiti M, Alexeeva E, Kostik M, Maggio M, Al Mayouf S, Saad C, Conti G, Gallizzi R, Civino A, Shimizu M, Felici E, Pistorio A, Ruperto N, Consolaro A, Ravelli A. Development and Initial Validation of the Systemic JADAS, a New Composite Disease Activity Score for Systemic Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/development-and-initial-validation-of-the-systemic-jadas-a-new-composite-disease-activity-score-for-systemic-juvenile-idiopathic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-initial-validation-of-the-systemic-jadas-a-new-composite-disease-activity-score-for-systemic-juvenile-idiopathic-arthritis/