Session Information
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose: Adverse events (AE) during treatment in RA patients are unavoidable. Monitoring AEs in real time during long-term treatment is critical for AE detection and rational management. The current AE report system in MedWatch can only collect rare AE, but could not support post marketing vigilance and reevaluation for drugs, especially in real world combination therapy.
To develop and validate SSDM as a new platform for post-marketing vigilance through monitoring AEs with common mono- and combination therapies in treatment of rheumatoid arthritis (RA).
Methods: The SSDM is a mobile App which includes two applications for both physicians and patients. Patients were educated to input the data of lab test records and treatment regiments and perform disease activity evaluation once a month. After data entry, patients can synchronize data to the mobile terminal of their authorized rheumatologist and upload onto cloud, which formed a large patient self-management database.
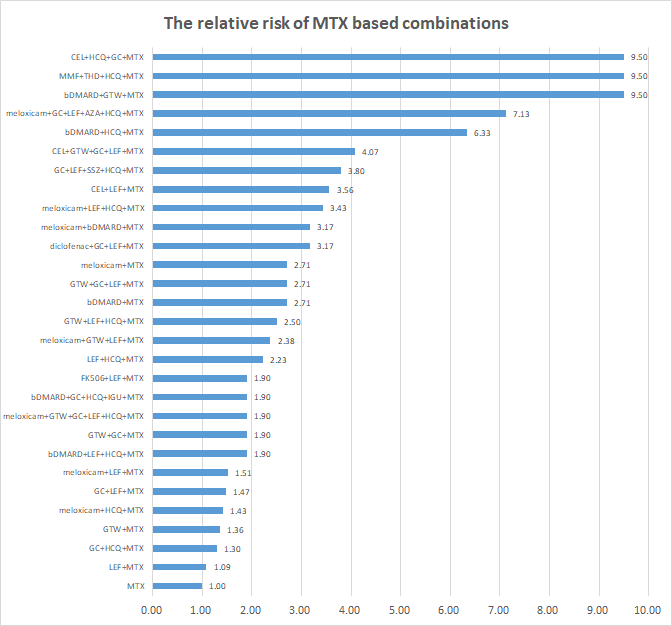
Results: From Aug 2014 to May 2018, a total of 10,903 RA patients from 480 centers in China were entered in the cohort study. These patients contributed more than 18,823 patient-years (PY) follow-up data. The mean age was 49.29 +/- 16.08 (18 to 99) years and the median disease duration was 27.4 months. The treatment regimens include 11 mono and 39 combination therapies. Taken ALT as an example, the profile of AE of ALT elevation for top ten mono therapy regimens is plot (Figure 1). In the database there are thirty-six MTX based combinations treatment regimens. The specifications of elevation of ALT in MTX combination therapy are drafted based on relative risk ratio comparing with rate in MTX mono therapy (Figure 2). Both AE profiles in mono therapy and specification in combination treatment alerted clinicians for unsafe regiments avoidance and timely adjustment.
Conclusion: The findings show that the AEs may be well collected and described via SSDM database through empowering patient. Rheumatologists are able to monitor the real world profile of adverse events in real time. SSDM is able to assist Rheumatologist in making rational clinical decision. SSDM may serve as new platform for post-marketing vigilance.
To cite this abstract in AMA style:
Wang Y, Wei H, Yang J, Li C, Mu R, Wu B, Wang H, Wang Y, Wang X, Guo H, Xue J, Ru J, Xin X, Zhang F, Kang L, Liu H, Liu X, Xie T, Shen L, Yang H, Li S, Li F, Wang F, Wu R, Liu Y, Xiao H, Jia Y, Xiao F, Huang J. Develop a New Platform for Post-Marketing Vigilance Though Smart System of Disease Management (SSDM) Mobiles Tools: A Real World Cohort Study of RA Patients from China [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/develop-a-new-platform-for-post-marketing-vigilance-though-smart-system-of-disease-management-ssdm-mobiles-tools-a-real-world-cohort-study-of-ra-patients-from-china/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/develop-a-new-platform-for-post-marketing-vigilance-though-smart-system-of-disease-management-ssdm-mobiles-tools-a-real-world-cohort-study-of-ra-patients-from-china/