Session Information
Date: Monday, November 13, 2023
Title: (1124–1154) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Deucravacitinib, a first-in-class, oral, selective, allosteric tyrosine kinase 2 inhibitor, is approved in multiple countries for the treatment of adults with plaque psoriasis. Deucravacitinib was superior to placebo and apremilast in the global, 52-week, phase 3 POETYK PSO-1 (NCT03624127) and PSO-2 (NCT03611751) trials in psoriasis. Deucravacitinib is being investigated in several immune-mediated diseases and has shown efficacy compared with placebo in phase 2 trials for SLE (NCT03252587) and PsA (NCT03881059). The POETYK long-term extension (LTE) trial (NCT04036435) showed that deucravacitinib maintained long-term efficacy through 2 years with no new safety signals. Here, we report clinical efficacy up to 3 years (148 weeks) in the POETYK LTE trial in a subset of patients who received continuous deucravacitinib from day 1 in the parent trials.
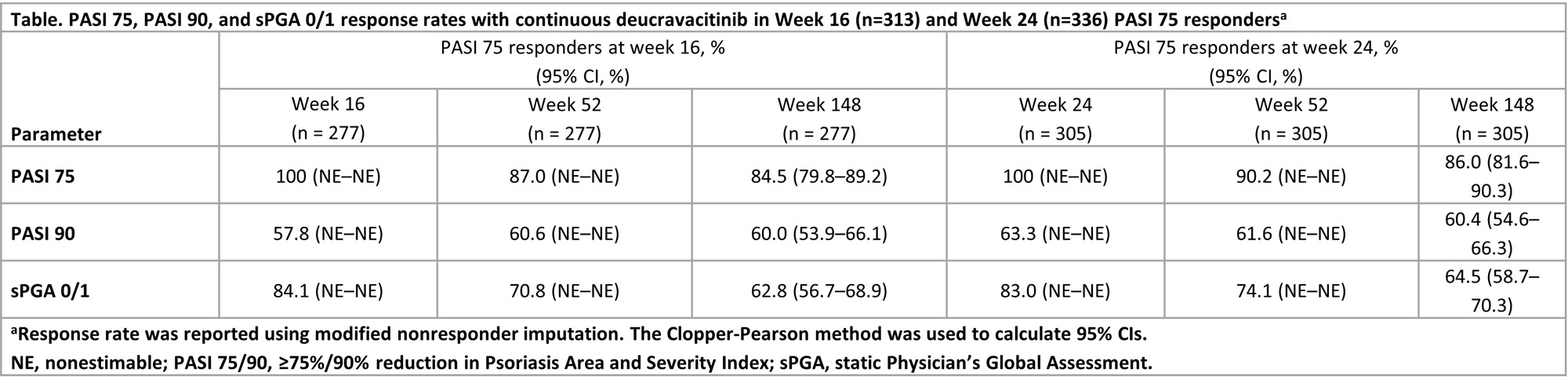
Methods: In POETYK PSO-1 and PSO-2, patients were randomized 1:2:1 to placebo, deucravacitinib 6 mg once daily (QD), or apremilast 30 mg twice daily. At week 52, patients could enter the POETYK LTE trial and receive open-label deucravacitinib 6 mg QD. Deucravacitinib efficacy through week 148 was evaluated in patients from pooled POETYK PSO-1 and PSO-2 populations who received continuous deucravacitinib from day 1, achieved ≥ 75% reduction from baseline in Psoriasis Area and Severity Index (PASI 75) at week 16 (primary endpoint) or week 24 (peak response), and enrolled in the POETYK LTE trial. Maintenance of response was assessed through data cutoff (June 15, 2022) and included PASI 75 and PASI 90 (≥ 90% reduction from baseline in PASI). Static Physician’s Global Assessment (sPGA) score of 0 (clear) or 1 (almost clear) with a ≥ 2-point improvement from baseline was assessed.
Results: A total of 513 patients completed 52 weeks in the parent trials and received continuous deucravacitinib from day 1, including 313 (61.4%) patients (95% CI, 57.0–65.6) who achieved PASI 75 at week 16 and 336 (66.5%) patients (95% CI, 62.2–70.6) who achieved PASI 75 at week 24. Among these patients, PASI 75 response rates were maintained from week 52 to week 148 (Table). PASI 90 response rates were maintained in > 50% of patients from the start of the POETYK LTE trial (Table). Response rates for sPGA 0/1 were maintained from week 52 to week 148 (Table).
Conclusion: Clinical efficacy was maintained for up to 148 weeks with continuous deucravacitinib in most patients who were week 16 and week 24 PASI 75 responders from the parent trials and enrolled in the POETYK LTE trial. These findings further support the long-term use of once-daily oral deucravacitinib as an effective treatment for patients with psoriasis.
To cite this abstract in AMA style:
Strober B, Sofen H, Imafuku S, Paul C, Gooderham M, Spelman L, Seo S, Passeron T, Kisa R, Berger V, Vritzali E, Hoyt K, Colombo M, Banerjee S, Augustin M, Stein Gold L, Alexis A, Thaçi D, Blauvelt A, Lebwohl M. Deucravacitinib in Plaque Psoriasis: Maintenance of Response over 3 Years [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/deucravacitinib-in-plaque-psoriasis-maintenance-of-response-over-3-years/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deucravacitinib-in-plaque-psoriasis-maintenance-of-response-over-3-years/