Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Tyrosine kinase 2 (TYK2) is an intracellular kinase in the Janus kinase (JAK) family that mediates the signaling of multiple cytokines, including those central to the immunopathogenesis of psoriatic arthritis (PsA), such as IL-23. Deucravacitinib (DEUC) is a novel, oral, selective, allosteric inhibitor of TYK2 that acts by binding to the unique regulatory domain on the enzyme. In a phase 2 trial in patients with active PsA, DEUC was significantly more efficacious than placebo (PBO) after 16 weeks of treatment and was well tolerated.1 This post-hoc analysis further evaluated the effect of DEUC on achievement of individual components of minimal disease activity (MDA) in patients through week 16 of the phase 2 PsA trial.
Methods: This double-blind, multicenter phase 2 trial (NCT03881059) enrolled patients (n=203) with a PsA diagnosis ≥6 months who fulfilled Classification Criteria for Psoriatic Arthritis at screening and had active joint disease (≥ 3 tender and ≥ 3 swollen joints), high-sensitivity C-reactive protein ≥ 3 mg/L, and ≥ 1 plaque psoriasis lesion (≥ 2 cm). Eligible patients had failed or were intolerant to ≥ 1 NSAID, conventional synthetic DMARD, and/or 1 tumor necrosis factor inhibitor (≤ 30%). Patients were randomized 1:1:1 to receive PBO, DEUC 6 mg once daily (QD), or DEUC 12 mg QD. The percentage of patients achieving MDA (5/7: tender joint count [TJC] ≤ 1, swollen joint count [SJC] ≤ 1, tender entheseal points [TEP] ≤ 1, Patient Global Assessment of Disease Activity [PtGA] ≤ 20, Patient Global Assessment of Pain [Pain] ≤ 15, Health Assessment Questionnaire-Disability Index [HAQ-DI] ≤ 0.5, and Psoriasis Area and Severity Index [PASI] ≤ 1 or body surface area [BSA] ≤ 3%) as well as proportions of patients achieving each of the MDA components in all patients, MDA responders, and nonresponders were assessed through week 16.
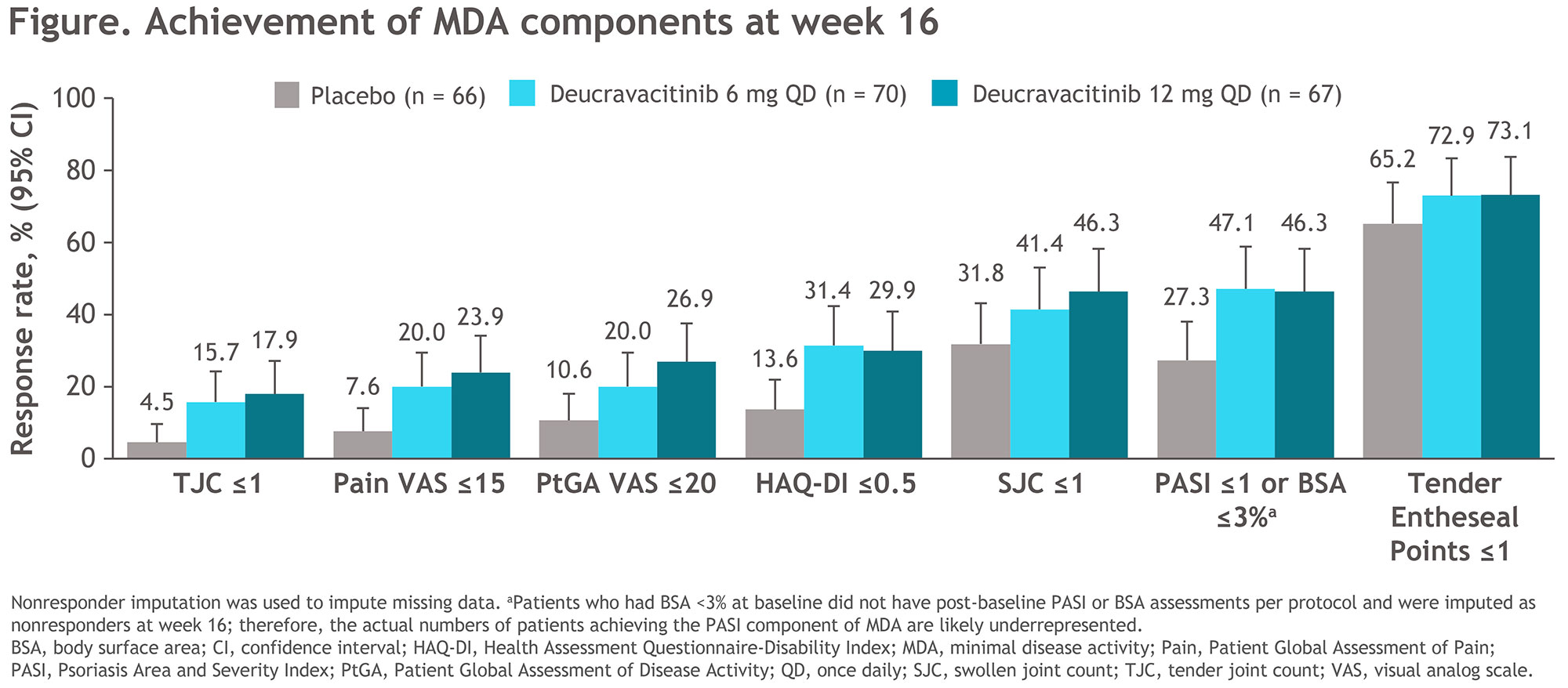
Results: Of 203 patients randomized, 180 (89%) completed 16 weeks of treatment (PBO, 58/66 [88%]; DEUC 6 mg QD, 63/70 [90%]; DEUC 12 mg QD, 59/67 [88%]). Demographic and baseline disease characteristics were generally similar across groups. Although no patient had met 5 of 7 criteria required for achieving MDA at baseline, several individual components of MDA were met at baseline, most frequently TEP ≤ 1 (PBO, 57.6%; DEUC 6 mg QD, 64.3%; DEUC 12 mg QD, 65.7%). After 16 weeks of treatment, 7.6%, 22.9%, and 23.9% of patients in the PBO, DEUC 6 mg QD, and DEUC 12 mg QD groups, respectively, achieved MDA response. Treatment with DEUC was associated with a numerically greater mean reduction from baseline in all MDA components versus PBO over 16 weeks of treatment in all patients. At week 16, more patients receiving DEUC versus PBO achieved the threshold for each of the MDA components (Figure).
Conclusion: After 16 weeks of treatment, patients receiving DEUC achieved a higher rate of MDA response compared with patients receiving PBO.
More patients receiving DEUC achieved each of the MDA components compared with patients receiving PBO.
Reference
1. Mease PJ, et al. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis. 2022;81:815-822.
To cite this abstract in AMA style:
Kavanaugh A, Coates L, Merola J, Mease P, Nowak M, Banerjee S, Hippeli L, Lehman T. Deucravacitinib, an Oral, Selective Tyrosine Kinase 2 Inhibitor, in a Phase 2 Trial in Psoriatic Arthritis: Achievement of Minimal Disease Activity and Its Components [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/deucravacitinib-an-oral-selective-tyrosine-kinase-2-inhibitor-in-a-phase-2-trial-in-psoriatic-arthritis-achievement-of-minimal-disease-activity-and-its-components/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deucravacitinib-an-oral-selective-tyrosine-kinase-2-inhibitor-in-a-phase-2-trial-in-psoriatic-arthritis-achievement-of-minimal-disease-activity-and-its-components/