Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Herpes Zoster (HZ) causes an infection commonly known as shingles. Patients with HZ are at increased risk for post-herpetic neuralgia, which is painful for patients and costly for the United States (US) healthcare system. Certain medications and diseases such as RA, SLE, and IBD weaken the immune system making the body more susceptible to HZ. Two vaccines are available in the US. Zostavax, a live-attenuated vaccine (ZVL), was approved by the FDA in 2006 and Shingrix, an adjuvanted recombinant zoster vaccine (RZV), was approved in 2017. ZVL reduces the risk of HZ by 65% in patients aged 50-59, and by 50% in patients aged 60-69. Despite the relative reduction of HZ with vaccination, rates in the general population remain low. Vaccination rates in 2016 were estimated at 33.4% among adults 60 years and older and 37.4% among adults 65 years and older.
The objective was to determine zoster vaccination rates of ZVL or RZV in patients on chronic immunosuppressive therapy at the Southeast Louisiana Veterans Healthcare System. We describe the correlation between vaccination rate and age, race, immunosuppressive medication and diagnosis.
Methods: This was a descriptive study with a retrospective review of records in the Computerized Patient Record System (CPRS). We included veterans 50 years and older treated with the following immunosuppressive medications: AZA, MMF, cyclosporine, MTX, LEF, tofacitinib, infliximab, adalimumab, etanercept, abatacept, rituximab or certolizumab from January 1, 2016 through December 31, 2018 (n= 601). Veterans with a history of malignancy, diabetes mellitus, HIV infection or death during the study period were excluded (n= 369). Charts were reviewed to determine receipt of ZVL or RZV and identify concurrent glucocorticoid therapy.
Results: 31 (13.4%) patients in our study population were vaccinated against HZ (Figure 1). Of the 232 patients included in the study, 66 (28.4%) were offered vaccination and 166 (71.6%) were not. Of the patients offered vaccination, 35 (53%) refused or had no documentation of receipt of vaccination. Of those vaccinated, 24 (77.4%) received RZV and 7 (22.6%) received ZVL.
Age and glucocorticoid therapy had a significant effect on whether vaccination was offered (Figure 2). Patients 70 years and older were 2.52 times more likely to be offered vaccination than patients less than 60 years of age (reference group). Patients on prednisone therapy were 2.26 times more likely to be offered vaccination than those not on prednisone therapy.
Patients on immunosuppressive therapy for gastrointestinal disorders were less likely to receive vaccination against HZ (Figure 3).
Race, gender and immunosuppressive medication did not influence the offer of vaccination against HZ in our study cohort.
Conclusion: The rate of vaccination against HZ in patients on immunosuppressive therapy in our study population was low despite the accessibility of vaccines. This is concerning given the risk of infection in this population and the proven efficacy of zoster vaccination. An extension of this study will explore factors that influencevaccination ratesand investigate interventions to improve vaccination rates. Interventions should include both provider and patient education.
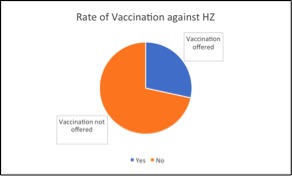 Figure 1: Rate of Vaccination against HZ
Figure 1: Rate of Vaccination against HZ
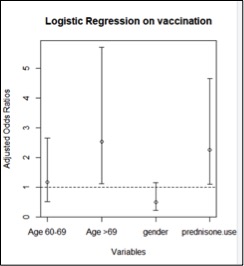 Figure 2: Vaccination Offered: Odds Ratios with 95% Confidence Interval
Figure 2: Vaccination Offered: Odds Ratios with 95% Confidence Interval
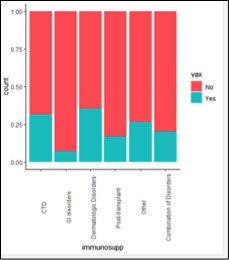 Figure 3: Rate vaccination was offered with respect to the diagnosis requiring immunosuppression
Figure 3: Rate vaccination was offered with respect to the diagnosis requiring immunosuppression
To cite this abstract in AMA style:
Kenninger H, Dayno R, Emejuaiwe N, Robledo-Vega I, Bembry W, Guevara M, Mahato S. Determining the Zoster Vaccination Rate Among Veterans on Chronic Immunosuppressive Therapy at the Southeast Louisiana Veterans Healthcare System – a Quality Indicator [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/determining-the-zoster-vaccination-rate-among-veterans-on-chronic-immunosuppressive-therapy-at-the-southeast-louisiana-veterans-healthcare-system-a-quality-indicator/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/determining-the-zoster-vaccination-rate-among-veterans-on-chronic-immunosuppressive-therapy-at-the-southeast-louisiana-veterans-healthcare-system-a-quality-indicator/
