Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient and family participation in research is critical to improving health outcomes, and identifying factors that contribute to participation or lack of participation in clinical trials is a necessary first step. There is limited data regarding why patients with lupus choose to participate in clinical trials. Research in other conditions has identified factors including age, race/ethnicity, gender, presence of co-morbidities, and disease severity. The primary objective of this study was to examine whether age, race/ethnicity, sex, or greater disease organ involvement are associated with previous clinical trial participation in patients with lupus.
Methods: Data were collected by The Lupus and Allied Diseases Association, the Lupus Foundation of America, and the Lupus Research Alliance for their Externally-led Patient-Focused Drug Development (PFDD) Initiative. From this dataset, we examined the influence of age, sex, race/ethnicity, and organ involvement on clinical trial participation. Data was available for 2,100 individuals with lupus or their parents/legal guardians for children. Participants completed a 46-question survey (in English or Spanish) electronically or on paper, which was distributed online or at lupus events. Logistic regression was used to test a model including demographic and disease characteristics; it was expanded to include duration of illness, number of medications, number of comorbidities, and number of other autoimmune diseases.
Results: Survey participants’ characteristics are summarized in Table 1. More than 50% of respondents had not participated in a clinical trial. Univariate analysis suggests that race/ethnicity, age of symptom onset, illness duration, lupus type, and history of organ transplant were associated with trial participation. The expanded model indicated that African Americans (OR=1.71) and Hispanics (OR=1.42) were more likely to participate than whites, respondents reporting longer illness duration were more likely to participate (OR=1.52), and respondents with a greater number of medications (OR=1.13) and other self-reported autoimmune diseases (OR=1.07) were more likely to participate in a clinical trial (Table 2).
Conclusion: Several medical (i.e., illness duration, number of medications and comorbid autoimmune disorder) and demographic (i.e., race/ethnicity) factors appear to be important determinants of participation in clinical trials. Compared to previous studies, these data suggest that African American and Hispanic individuals are willing to participate in clinical trials, but perhaps only trials that are related to their chronic condition, or this finding may be due to underrepresentation in this sample. Future research studies with larger samples of diverse participants are recommended.
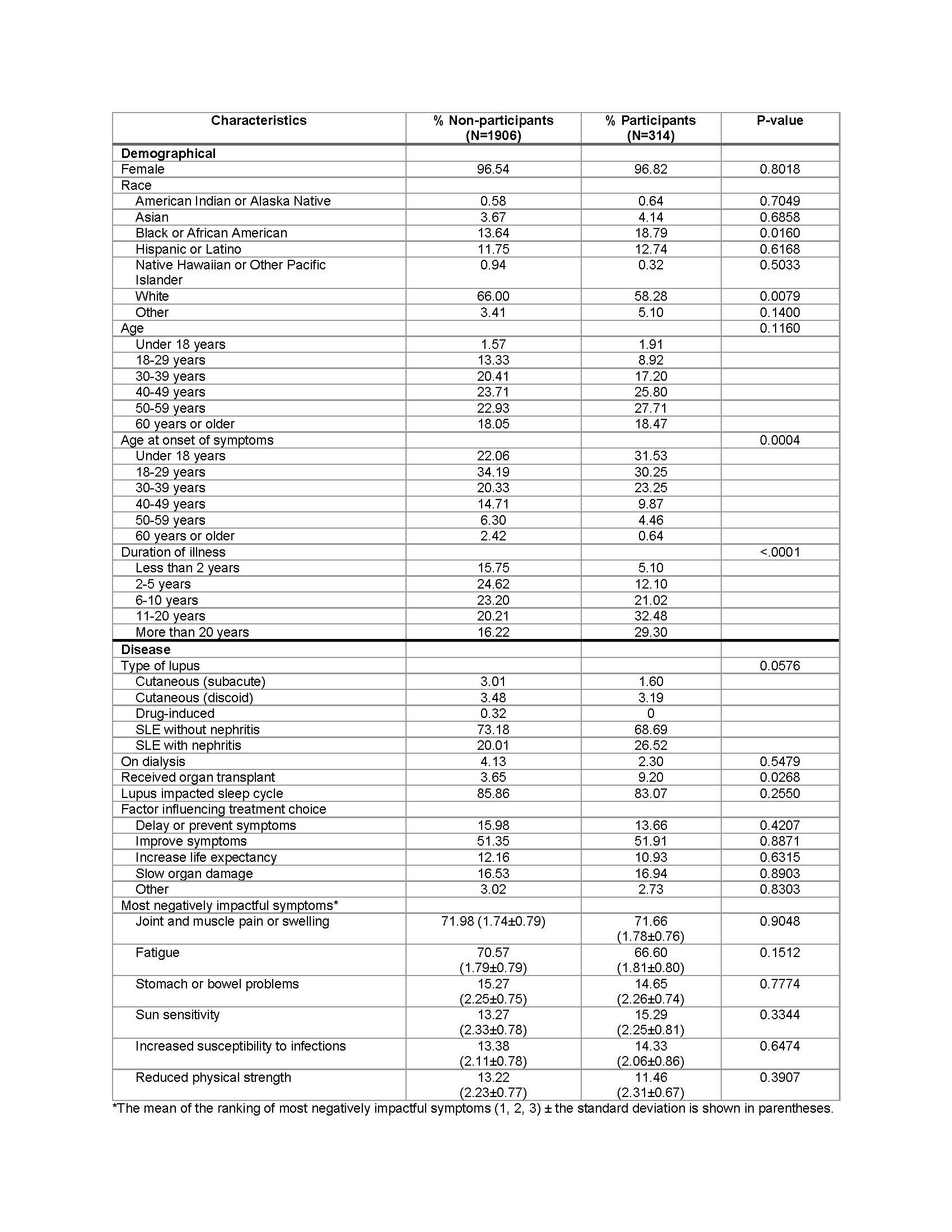 Table 1. Demographic and disease characteristics of clinical trial non-participants vs. participants
Table 1. Demographic and disease characteristics of clinical trial non-participants vs. participants
 Table 2. Multivariate logistic regression model showing effect of different variables (e.g., age (on ordinal scale), gender, race) on clinical trial participation
Table 2. Multivariate logistic regression model showing effect of different variables (e.g., age (on ordinal scale), gender, race) on clinical trial participation
To cite this abstract in AMA style:
Harry O, Langefeld C, Marion M, Younts T, Crosby L, Vitolins M, Modi A. Determinants of Participation in Clinical Trials Among Patients with Lupus in the United States [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/determinants-of-participation-in-clinical-trials-among-patients-with-lupus-in-the-united-states/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/determinants-of-participation-in-clinical-trials-among-patients-with-lupus-in-the-united-states/
