Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: This study was conducted to uncover real-world treatment patterns among moderate to severely active systemic lupus erythematosus (SLE) patients in the US.
Methods: 1,011 records from SLE patients with moderate and severely active disease were collected in collaboration with 297 US rheumatologists via an online survey platform from March 31 through May 5, 2023.
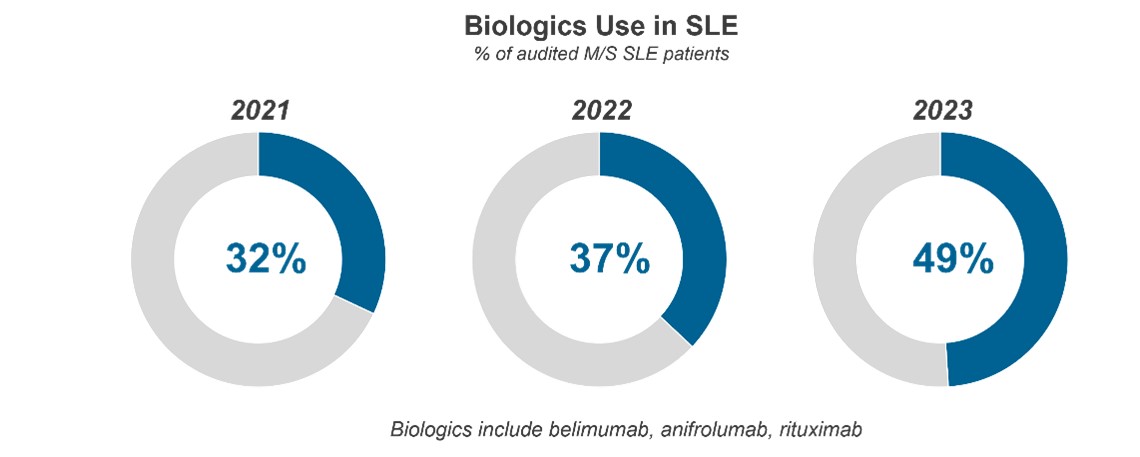
Results: Study results reveal that the use of biologics in SLE has substantially increased over the past three years – marking a dramatic expansion of biologic use in moderate-to-severe SLE in a relatively short period of time.
37% of audited patients are currently on FDA-approved biologic belimumab for their moderate-to-severe SLE, substantially up from levels observed in 2022 (28%) and 2021 (21%) and increasingly driven by the drug’s anticipated efficacy, steroid-sparing ability, safety profile, and recent lupus nephritis (LN) indication. 8% of audited moderate-to-severe patients are currently on FDA-approved biologic anifrolumab compared to 3% noted in the 2022 audit.* Like belimumab, rheumatologists were most compelled to prescribe anifrolumab due to its perceived efficacy, steroid-sparing effect, and safety profile.
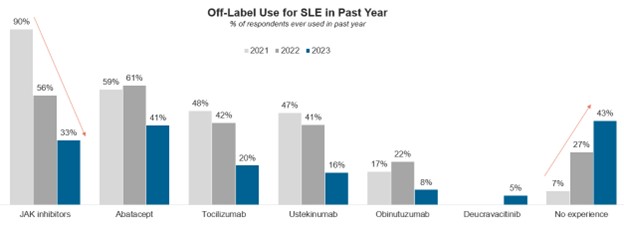
Commonly used “off label” biologic rituximab, on the other hand, is seeing a year-over-year decline in patient share with rheumatologists (4% of moderate-to-severe patients in 2023, 6% in 2022 and 11% in 2021) and physicians are considering fewer of their patients as potential candidates for the drug.
Notably, rheumatologists have also been using fewer other off-label agents in SLE over the same period, likely due to the availability of recently approved SLE agents along with safety concerns associated with the Janus kinase (JAK) inhibitor class, which has shown a steep decline in off-label use for SLE since 2021.
While the majority of rheumatologists express satisfaction with patients’ response to approved biologics belimumab and anifrolumab, it is notable that only 18% of rheumatologists agree with the statement “I am satisfied with the treatment options available to me in SLE,” underscoring the remaining unmet need for new pharmacologic treatment options.
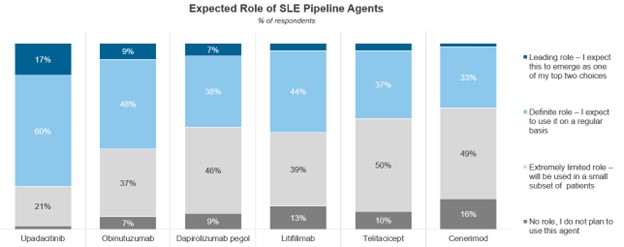
The robust SLE pipeline – which consists of over 40 drugs in development – provides promising future treatment options for SLE. Of the SLE pipeline agents in or entering Phase III, rheumatologists expect upadacitinib to play the most important role if approved with 77% of rheumatologists saying the drug will play a leading or definite role.
While rheumatologists have been lessening their use of off-label JAK inhibitors in SLE, upadacitinib is a drug rheumatologists have experience using in a host of other rheumatic conditions, which could revitalize use of JAKs in SLE if approved.
Conclusion: Rheumatologists are increasingly using approved biologics in their moderate-to-severe SLE patients, however an unmet need for new pharmacological treatments remains. Despite the lessening of off label use of the JAK class many rheumatologists believe upadacitinib could provide significant value to their treatment armamentarium if approved.
To cite this abstract in AMA style:
Rex R, Yarnall M, May S. Despite Dramatic Expansion of Approved Biologics in SLE, Unmet Needs Remain [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/despite-dramatic-expansion-of-approved-biologics-in-sle-unmet-needs-remain/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/despite-dramatic-expansion-of-approved-biologics-in-sle-unmet-needs-remain/