Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: The use of a control arm (comparator) in randomized controlled trials (RCTs) is considered the gold standard for trial conduct and interpretation. Choosing an appropriate control for RCTs evaluating Cognitive Behavioral Therapy (CBT) interventions is particularly challenging, as it is not feasible to develop a blinded sham behavioral treatment. Pilot and pivotal RCTs were performed to evaluate the efficacy of a smartphone based digital therapeutic app (FM-ACT), which delivers self-guided Acceptance and Commitment Therapy (ACT) for treatment of fibromyalgia symptoms. For the control arm of these RCTs, a digital comparator app was developed. The comparator app was designed to offer similar participant engagement and usability to control for potential study biases. This report presents the approach and validation of the novel comparator design.
Methods: In both RCTs, participants meeting 2016 diagnostic criteria for fibromyalgia were randomized to receive 12 weeks of FM-ACT or the digital symptom tracker (ST) comparator. The FM-ACT intervention consists of 42 daily sessions of structured ACT lessons, mindfulness practices, and activities to encourage paced exercise and behavior change. The ST app was developed as an alternative intervention consisting of two common pain management components: 1) patient education on FM and general health; and 2) daily symptom and function tracking with progress reports available to the participant. During consent, participants were informed that they would be evaluating one of two potentially effective digital treatments. Participant engagement (number of sessions completed during the study) and usability factors were assessed to compare the performance of this novel control strategy.
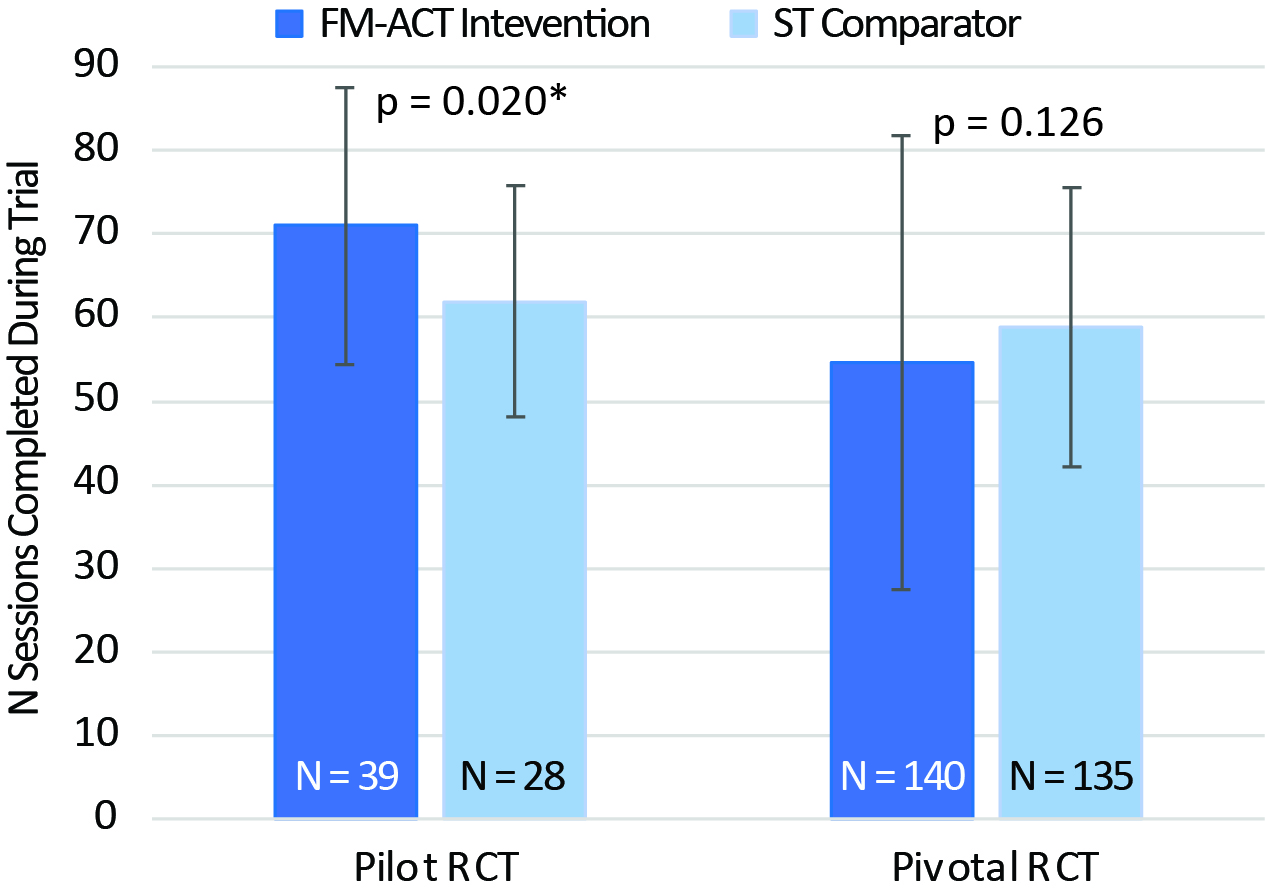
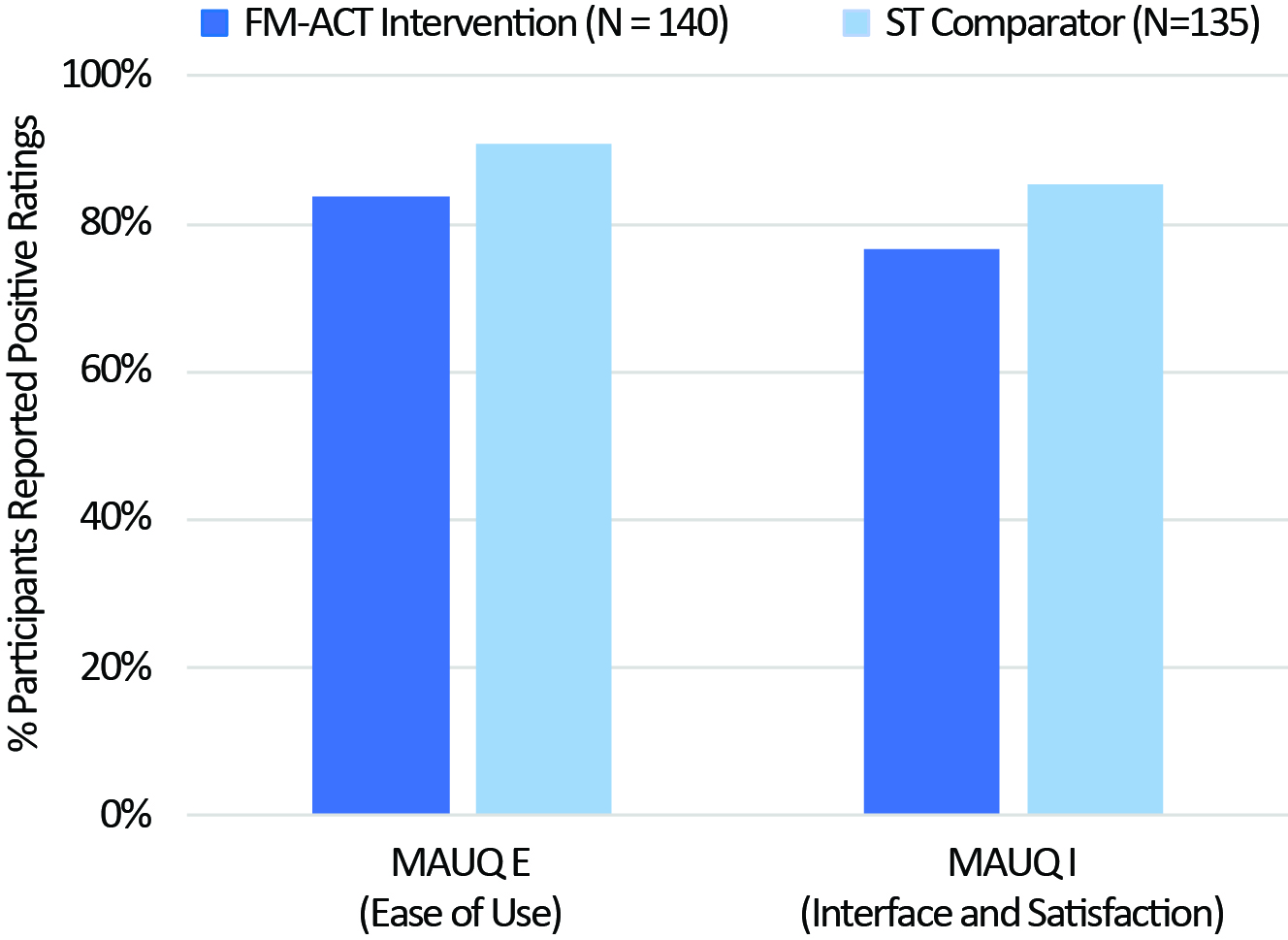
Results: The pilot RCT data demonstrated a similar number of completed sessions between the FM-ACT and ST comparator arms, which was further supported by the results from the pivotal RCT (Fig. 1). Usability assessment from the pivotal trial showed more participants reported “good” to “excellent” usability comparing the ST arm to FM-ACT (Fig. 2).
Participants from both arms reported high rates of satisfaction with their app experience (proportion of satisfied participants: FM-ACT 80%, ST: 85%). Similar proportion of participants from both arms reported that they would use the app again (FM-ACT 80%, ST 79%).
Conclusion: Participants randomized to the ST comparator arm engaged with the app at rates equal to or exceeding FM-ACT engagement rates and had similar usability ratings. The evidence suggests that the ST design may minimize participant bias towards a perceived inferior therapy. This approach may provide a basis for comparator strategies for RCTs of behavioral health interventions.
*Although statistical significance was found in the pilot RCT, both arms exhibited high app engagement with 97.3% and 92.6% of the participants completed ≥ 42 sessions (corresponding to the core therapy content) in the FM-ACT and ST arm, respectively.
To cite this abstract in AMA style:
Gendreau M, Dai Y, Rosenbluth M, Williams D, Clauw D. Design and Validation of a Comparator for Randomized Controlled Trials of a Digital Cognitive Behavioral Therapy [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/design-and-validation-of-a-comparator-for-randomized-controlled-trials-of-a-digital-cognitive-behavioral-therapy/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/design-and-validation-of-a-comparator-for-randomized-controlled-trials-of-a-digital-cognitive-behavioral-therapy/