Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Between 2000 and 2006, the only other treatment option for patients with rheumatoid arthritis (RA) first exposed to an anti-TNF agent was another anti-TNF agent. After 2006, agents with a different mode of action became available. The patterns of switches (anti-TNF to anti-TNF) and swaps (anti-TNF to another mode of action) have not been presented in detail. The real-world experience gathered since 2000 gives us a unique opportunity to examine change in prescribing patterns.
Methods: The data of biologic naïve RA patients starting a first anti-TNF [adalimumab (ADA), certolizumab (CER), etanercept (ETA), golimumab (GOL), infliximab (INF) or inflectra] between January 1, 2000 and December 31,2006 (first era), and January 1, 2007 and December 2015 (second era) was extracted. Patients were followed until they stopped, changed their medication or were lost to follow-up. Switches or swaps could happen at any time. We analyzed the number of patients that stopped, switched or swapped in each treatment era and the time from initial treatment to second treatment or the cessation of treatment. Statistical analysis was performed using SAS version 9.4.
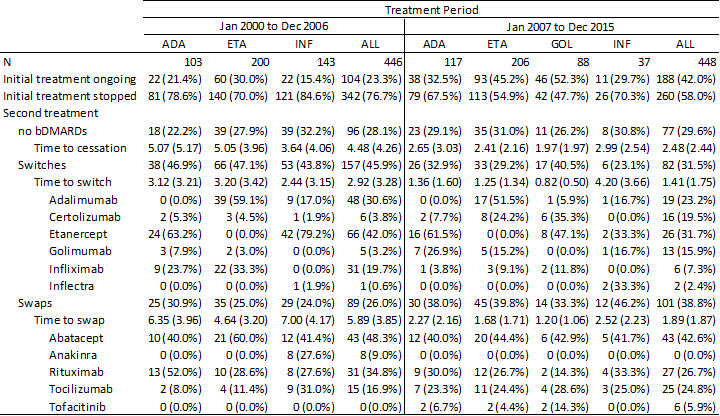
Results: The choice of a second biologic agent among patients having been prescribed their initial between Jan 1, 2000, and Dec 31, 2006, and Jan 1, 2007, and Dec 31, 2015, are presented Table 1. In the first era, switches accounted for 45.9% of cases, with most switching to ETN (42%), followed by ADA (30.6%), INF (19.7%), CER (3.8%), GOL (3.2%) and inflectra (0.6%). In the second era, switches accounted for 31.5% of cases, with most switching to ETN (31.7%), ADA (23.2%), CER (19.5%), GOL (15.9%), INF (7.3%) and inflectra (2.4%). Swaps accounted for 26.0% of cases in the first era. These swaps occurred towards abatacept (ABA), rituximab (RTX), tocilizumab (TOCI) and anakinra in 48.3%, 34.8%, 16.9% and 9.0% of cases. In the second era, the proportion of swaps increased to 38.8% and occurred towards ABA, RTX, TOCI and tofacitinib (TOFA) accounting for 42.6%, 26.7%, 24.8% and 5.9% of cases.
Conclusion: When switching occurs, most patients switched to a second anti-TNF in the first and second era. ETN was the anti-TNF more often selected for the in both eras. In the second era, there was an increased tendency to prescribe agents with a different mode of action after the first anti-TNF agent. This may be due to the availability of biologics with a different mode of action.
Table 1.
To cite this abstract in AMA style:
Choquette D, Bessette L, Coupal L, Garces K. Descriptive Patterns of Switches and Swaps in Patients with Rheumatoid Arthritis Initially Treated with Anti-TNF Agents in First Intention between 2000 and 2006, and 2007 and 2015 – Experience from a Real-World Database RHUMADATA® [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/descriptive-patterns-of-switches-and-swaps-in-patients-with-rheumatoid-arthritis-initially-treated-with-anti-tnf-agents-in-first-intention-between-2000-and-2006-and-2007-and-2015-experience-from-a/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/descriptive-patterns-of-switches-and-swaps-in-patients-with-rheumatoid-arthritis-initially-treated-with-anti-tnf-agents-in-first-intention-between-2000-and-2006-and-2007-and-2015-experience-from-a/